Methocinnamox Reverses and Prevents Fentanyl-Induced Ventilatory Depression in Rats
- PMID: 33431611
- PMCID: PMC7985616
- DOI: 10.1124/jpet.120.000387
Methocinnamox Reverses and Prevents Fentanyl-Induced Ventilatory Depression in Rats
Abstract
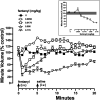
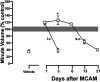
Opioid use disorder affects over 2 million Americans with an increasing number of deaths due to overdose from the synthetic opioid fentanyl and its analogs. The Food and Drug Administration-approved opioid receptor antagonist naloxone (e.g., Narcan) is used currently to treat overdose; however, a short duration of action limits its clinical utility. Methocinnamox (MCAM) is a long-lasting opioid receptor antagonist that may reverse and prevent the ventilatory-depressant effects of fentanyl. This study compared the ability of naloxone (0.0001-10 mg/kg) and MCAM (0.0001-10 mg/kg) to reverse and prevent ventilatory depression by fentanyl and compared the duration of action of MCAM intravenously and subcutaneously in two procedures: ventilation and warm-water tail withdrawal. In male Sprague-Dawley rats (N = 8), fentanyl (0.0032-0.178 mg/kg, i.v.) decreased minute volume in a dose- and time-dependent manner with a dose of 0.178 mg/kg decreasing VE to less than 40% of control. MCAM and naloxone reversed the ventilatory-depressant effects of 0.178 mg/kg fentanyl in a dose-related manner. The day after antagonist administration, MCAM but not naloxone attenuated the ventilatory-depressant effects of fentanyl. The duration of action of MCAM lasted up to 3 days and at least 2 weeks after intravenous and subcutaneous administration, respectively. MCAM attenuated the antinociceptive effects of fentanyl, with antagonism lasting up to 5 days and more than 2 weeks after intravenous and subcutaneous administration, respectively. Reversal and prolonged antagonism by MCAM might provide an effective treatment option for the opioid crisis, particularly toxicity from fentanyl and related highly potent analogs. SIGNIFICANCE STATEMENT: This study demonstrates that like naloxone, methocinnamox (MCAM) reverses the ventilatory-depressant effects of fentanyl in a time- and dose-related manner. However, unlike naloxone, the duration of action of MCAM was greater than 2 weeks when administered subcutaneously and up to 5 days when administered intravenously. These data suggest that MCAM might be particularly useful for rescuing individuals from opioid overdose, including fentanyl overdose, as well as protecting against the reemergence of ventilatory depression (renarconization).
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Figures





References
-
- Algera MH, Kamp J, van der Schrier R, van Velzen M, Niesters M, Aarts L, Dahan A, Olofsen E (2019) Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal. Br J Anaesth 122:e168–e179. - PubMed
-
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P (2018) Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin 34:573–576. - PubMed
-
- Bell A, Bennett AS, Jones TS, Doe-Simkins M, Williams LD (2019) Amount of naloxone used to reverse opioid overdoses outside of medical practice in a city with increasing illicitly manufactured fentanyl in illicit drug supply. Subst Abus 40:52–55. - PubMed
-
- Broadbear JH, Sumpter TL, Burke TF, Husbands SM, Lewis JW, Woods JH, Traynor JR (2000) Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinociception in the mouse: comparison with clocinnamox, β-funaltrexamine, and β-chlornaltrexamine. J Pharmacol Exp Ther 294:933–940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

