A link between synaptic plasticity and reorganization of brain activity in Parkinson's disease
- PMID: 33431672
- PMCID: PMC7826364
- DOI: 10.1073/pnas.2013962118
A link between synaptic plasticity and reorganization of brain activity in Parkinson's disease
Abstract
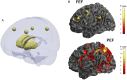
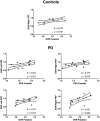
The link between synaptic plasticity and reorganization of brain activity in health and disease remains a scientific challenge. We examined this question in Parkinson's disease (PD) where functional up-regulation of postsynaptic D2 receptors has been documented while its significance at the neural activity level has never been identified. We investigated cortico-subcortical plasticity in PD using the oculomotor system as a model to study reorganization of dopaminergic networks. This model is ideal because this system reorganizes due to frontal-to-parietal shifts in blood oxygen level-dependent (BOLD) activity. We tested the prediction that functional activation plasticity is associated with postsynaptic dopaminergic modifications by combining positron emission tomography/functional magnetic resonance imaging to investigate striatal postsynaptic reorganization of dopamine D2 receptors (using 11C-raclopride) and neural activation in PD. We used covariance (connectivity) statistics at molecular and functional levels to probe striato-cortical reorganization in PD in on/off medication states to show that functional and molecular forms of reorganization are related. D2 binding across regions defined by prosaccades showed increased molecular connectivity between both caudate/putamen and hyperactive parietal eye fields in PD in contrast with frontal eye fields in controls, in line with the shift model. Concerning antisaccades, parietal-striatal connectivity dominated in again in PD, unlike frontal regions. Concerning molecular-BOLD covariance, a striking sign reversal was observed: PD patients showed negative frontal-putamen functional-molecular associations, consistent with the reorganization shift, in contrast with the positive correlations observed in controls. Follow-up analysis in off-medication PD patients confirmed the negative BOLD-molecular correlation. These results provide a link among BOLD responses, striato-cortical synaptic reorganization, and neural plasticity in PD.
Keywords: functional connectivity; functional magnetic resonance imaging; molecular imaging; positron emission tomography.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Chaudhuri K. R., Healy D. G., Schapira A. H.; National Institute for Clinical Excellence , Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 5, 235–245 (2006). - PubMed
-
- Lees A. J., Hardy J., Revesz T., Parkinson’s disease. Lancet 373, 2055–2066 (2009). - PubMed
-
- MacAskill M. R., Anderson T. J., Eye movements in neurodegenerative diseases. Curr. Opin. Neurol. 29, 61–68 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

