Interplay between desmoglein2 and hypoxia controls metastasis in breast cancer
- PMID: 33431674
- PMCID: PMC7826351
- DOI: 10.1073/pnas.2014408118
Interplay between desmoglein2 and hypoxia controls metastasis in breast cancer
Abstract
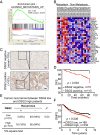
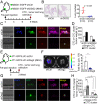
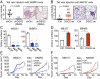
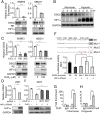
Metastasis is the major cause of cancer death. An increased level of circulating tumor cells (CTCs), metastatic cancer cells that have intravasated into the circulatory system, is particularly associated with colonization of distant organs and poor prognosis. However, the key factors required for tumor cell dissemination and colonization remain elusive. We found that high expression of desmoglein2 (DSG2), a component of desmosome-mediated intercellular adhesion complexes, promoted tumor growth, increased the prevalence of CTC clusters, and facilitated distant organ colonization. The dynamic regulation of DSG2 by hypoxia was key to this process, as down-regulation of DSG2 in hypoxic regions of primary tumors led to elevated epithelial-mesenchymal transition (EMT) gene expression, allowing cells to detach from the primary tumor and undergo intravasation. Subsequent derepression of DSG2 after intravasation and release of hypoxic stress was associated with an increased ability to colonize distant organs. This dynamic regulation of DSG2 was mediated by Hypoxia-Induced Factor1α (HIF1α). In contrast to its more widely observed function to promote expression of hypoxia-inducible genes, HIF1α repressed DSG2 by recruitment of the polycomb repressive complex 2 components, EZH2 and SUZ12, to the DSG2 promoter in hypoxic cells. Consistent with our experimental data, DSG2 expression level correlated with poor prognosis and recurrence risk in breast cancer patients. Together, these results demonstrated the importance of DSG2 expression in metastasis and revealed a mechanism by which hypoxia drives metastasis.
Keywords: HIF1α; breast cancer; circulating tumor cells (CTCs); desmoglein2 (DSG2); metastasis.
Conflict of interest statement
Competing interest statement: P.-H.C. and M.-C.C. are coinventors on a DSG2 monoclonal antibody patent owned by Asclepiumm Taiwan Co., Ltd. M.-C.C. has equity ownership in and serves on the board of directors of Asclepiumm Taiwan Co., Ltd.
Figures


References
-
- Chaffer C. L., Weinberg R. A., A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011). - PubMed
-
- Theodoropoulos P. A., et al. , Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 288, 99–106 (2010). - PubMed
-
- Cohen S. J., et al. , Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

