Invariant timescale hierarchy across the cortical somatosensory network
- PMID: 33431695
- PMCID: PMC7826380
- DOI: 10.1073/pnas.2021843118
Invariant timescale hierarchy across the cortical somatosensory network
Erratum in
-
Correction for Rossi-Pool et al., Invariant timescale hierarchy across the cortical somatosensory network.Proc Natl Acad Sci U S A. 2023 Mar 21;120(12):e2302619120. doi: 10.1073/pnas.2302619120. Epub 2023 Mar 15. Proc Natl Acad Sci U S A. 2023. PMID: 36920930 Free PMC article. No abstract available.
Abstract
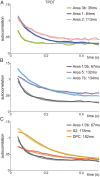
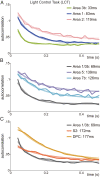
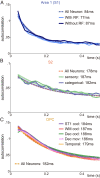
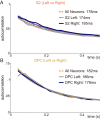
The ability of cortical networks to integrate information from different sources is essential for cognitive processes. On one hand, sensory areas exhibit fast dynamics often phase-locked to stimulation; on the other hand, frontal lobe areas with slow response latencies to stimuli must integrate and maintain information for longer periods. Thus, cortical areas may require different timescales depending on their functional role. Studying the cortical somatosensory network while monkeys discriminated between two vibrotactile stimulus patterns, we found that a hierarchical order could be established across cortical areas based on their intrinsic timescales. Further, even though subareas (areas 3b, 1, and 2) of the primary somatosensory (S1) cortex exhibit analogous firing rate responses, a clear differentiation was observed in their timescales. Importantly, we observed that this inherent timescale hierarchy was invariant between task contexts (demanding vs. nondemanding). Even if task context severely affected neural coding in cortical areas downstream to S1, their timescales remained unaffected. Moreover, we found that these time constants were invariant across neurons with different latencies or coding. Although neurons had completely different dynamics, they all exhibited comparable timescales within each cortical area. Our results suggest that this measure is demonstrative of an inherent characteristic of each cortical area, is not a dynamical feature of individual neurons, and does not depend on task demands.
Keywords: behaving monkeys; inherent time constants; primary somatosensory cortex; somatosensory network; timescale hierarchy.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

