Early impact of donor CYP3A5 genotype and Graft-to-Recipient Weight Ratio on tacrolimus pharmacokinetics in pediatric liver transplant patients
- PMID: 33432012
- PMCID: PMC7801660
- DOI: 10.1038/s41598-020-79574-7
Early impact of donor CYP3A5 genotype and Graft-to-Recipient Weight Ratio on tacrolimus pharmacokinetics in pediatric liver transplant patients
Abstract
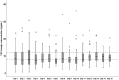
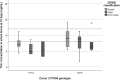
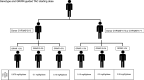
Tacrolimus (TAC) pharmacokinetics is influenced by the donor CYP3A5 genotype and the age of pediatric liver recipients. However, an optimization of a genotype-based algorithm for determining TAC starting is needed to earlier achieve stable target levels. As the graft itself is responsible for its metabolism, the Graft-to-Recipient Weight Ratio (GRWR) might play a role in TAC dose requirements. A single-center study was carried out in a cohort of 49 pediatric recipients to analyse the impact of patient and graft characteristics on TAC pharmacokinetics during the first 15 post-transplant days. Children < 2 years received grafts with a significantly higher GRWR (4.2%) than children between 2-8 (2.6%) and over 8 (2.7%). TAC concentration/weight-adjusted dose ratio was significantly lower in recipients from CYP3A5*1/*3 donors or with extra-large (GRWR > 5%) or large (GRWR 3-5%) grafts. The donor CYP3A5 genotype and GRWR were the only significant predictors of the TAC weight adjusted doses. Patients with a GRWR > 4% had a higher risk of acute rejection, observed in 20/49 (41%) patients. In conclusion, TAC starting dose could be guided according to the donor CYP3A5 genotype and GRWR, allowing for a quicker achievement of target concentrations and eventually reducing the risk of rejection.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
GRWR Correlates with the Metabolism of Tacrolimus after Pediatric Living Donor Liver Transplantation According to Donor CYP3A5 Polymorphism.Biomed Res Int. 2022 Oct 30;2022:7647754. doi: 10.1155/2022/7647754. eCollection 2022. Biomed Res Int. 2022. PMID: 36349313 Free PMC article.
-
Impact of donor CYP3A5 genotype on pharmacokinetics of tacrolimus in South African paediatric liver transplant patients.S Afr Med J. 2024 Apr 24;114(3b):e1367. doi: 10.7196/SAMJ.2024.v114i3b.1367. S Afr Med J. 2024. PMID: 39041443
-
Liver Transplant Patient Carriers of Polymorphism Cyp3a5*1 Donors May Need More Doses of Tacrolimus From the First Month After Transplantation.Transplant Proc. 2015 Oct;47(8):2388-92. doi: 10.1016/j.transproceed.2015.09.024. Transplant Proc. 2015. PMID: 26518936
-
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II.Clin Pharmacokinet. 2010 Apr;49(4):207-21. doi: 10.2165/11317550-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20214406 Review.
-
Evaluating the evidence for genotype-informed Bayesian dosing of tacrolimus in children undergoing solid organ transplantation: A systematic literature review.Br J Clin Pharmacol. 2024 Nov;90(11):2724-2741. doi: 10.1111/bcp.16203. Epub 2024 Aug 15. Br J Clin Pharmacol. 2024. PMID: 39147586
Cited by
-
Authors' Reply to Reed and Colleagues' Comment on "Role of mTOR Inhibitors in Pediatric Liver Transplant Recipients: A Systematic Review".Paediatr Drugs. 2025 Sep;27(5):667-669. doi: 10.1007/s40272-025-00704-7. Epub 2025 Jun 11. Paediatr Drugs. 2025. PMID: 40498299 No abstract available.
-
GRWR Correlates with the Metabolism of Tacrolimus after Pediatric Living Donor Liver Transplantation According to Donor CYP3A5 Polymorphism.Biomed Res Int. 2022 Oct 30;2022:7647754. doi: 10.1155/2022/7647754. eCollection 2022. Biomed Res Int. 2022. PMID: 36349313 Free PMC article.
-
The Effect of Voriconazole on Tacrolimus in Kidney Transplantation Recipients: A Real-World Study.Pharmaceutics. 2022 Dec 7;14(12):2739. doi: 10.3390/pharmaceutics14122739. Pharmaceutics. 2022. PMID: 36559231 Free PMC article.
-
Monitoring Tacrolimus Concentrations in Whole Blood and Peripheral Blood Mononuclear Cells: Inter- and Intra-Patient Variability in a Cohort of Pediatric Patients.Front Pharmacol. 2021 Nov 5;12:750433. doi: 10.3389/fphar.2021.750433. eCollection 2021. Front Pharmacol. 2021. PMID: 34803692 Free PMC article.
References
-
- Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab. Dispos. 1992;20:753–761. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

