Seasonal variation in UVA light drives hormonal and behavioural changes in a marine annelid via a ciliary opsin
- PMID: 33432133
- PMCID: PMC7611595
- DOI: 10.1038/s41559-020-01356-1
Seasonal variation in UVA light drives hormonal and behavioural changes in a marine annelid via a ciliary opsin
Abstract
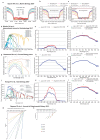
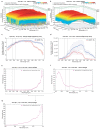
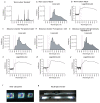
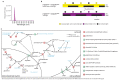
The right timing of animal physiology and behaviour ensures the stability of populations and ecosystems. To predict anthropogenic impacts on these timings, more insight is needed into the interplay between environment and molecular timing mechanisms. This is particularly true in marine environments. Using high-resolution, long-term daylight measurements from a habitat of the marine annelid Platynereis dumerilii, we found that temporal changes in ultraviolet A (UVA)/deep violet intensities, more than longer wavelengths, can provide annual time information, which differs from annual changes in the photoperiod. We developed experimental set-ups that resemble natural daylight illumination conditions, and automated, quantifiable behavioural tracking. Experimental reduction of UVA/deep violet light (approximately 370-430 nm) under a long photoperiod (16 h light and 8 h dark) significantly decreased locomotor activities, comparable to the decrease caused by a short photoperiod (8 h light and 16 h dark). In contrast, altering UVA/deep violet light intensities did not cause differences in locomotor levels under a short photoperiod. This modulation of locomotion by UVA/deep violet light under a long photoperiod requires c-opsin1, a UVA/deep violet sensor employing Gi signalling. C-opsin1 also regulates the levels of rate-limiting enzymes for monogenic amine synthesis and of several neurohormones, including pigment-dispersing factor, vasotocin (vasopressin/oxytocin) and neuropeptide Y. Our analyses indicate a complex inteplay between UVA/deep violet light intensities and photoperiod as indicators of annual time.
Conflict of interest statement
Competing interest statement: Max Hofbauer is the CEO of loopbio GmbH, a company developing commercial animal behavioral tracking solutions. C.M. is the CEO of Marine Breeding Systems GmbH, a company developing commercial illumination systems for aquaculture.
All other authors declare no conflict of interest.
Figures










Comment in
-
Timing requires the right amount and type of light.Nat Ecol Evol. 2021 Feb;5(2):153-154. doi: 10.1038/s41559-020-01373-0. Nat Ecol Evol. 2021. PMID: 33432135 No abstract available.
References
-
- Korringa P. Relations between the moon and periodicity in the breeding of marine animals. Ecological Monographs. 1947;17:347–381.
-
- Numata H, Helm B. Annual, lunar, and tidal clocks : patterns and mechanisms of nature’s enigmatic rhythms. Springer; 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

