Role of FMRP in rapid antidepressant effects and synapse regulation
- PMID: 33432187
- PMCID: PMC8440195
- DOI: 10.1038/s41380-020-00977-z
Role of FMRP in rapid antidepressant effects and synapse regulation
Abstract
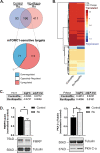
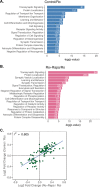
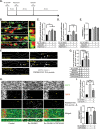
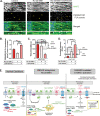
Rapid antidepressants are novel treatments for major depressive disorder (MDD) and work by blocking N-methyl-D-aspartate receptors (NMDARs), which, in turn, activate the protein synthesis pathway regulated by mechanistic/mammalian target of rapamycin complex 1 (mTORC1). Our recent work demonstrates that the RNA-binding protein Fragile X Mental Retardation Protein (FMRP) is downregulated in dendrites upon treatment with a rapid antidepressant. Here, we show that the behavioral effects of the rapid antidepressant Ro-25-6981 require FMRP expression, and treatment promotes differential mRNA binding to FMRP in an mTORC1-dependent manner. Further, these mRNAs are identified to regulate transsynaptic signaling. Using a novel technique, we show that synapse formation underlying the behavioral effects of Ro-25-6981 requires GABABR-mediated mTORC1 activity in WT animals. Finally, we demonstrate that in an animal model that lacks FMRP expression and has clinical relevance for Fragile X Syndrome (FXS), GABABR activity is detrimental to the effects of Ro-25-6981. These effects are rescued with the combined therapy of blocking GABABRs and NMDARs, indicating that rapid antidepressants alone may not be an effective treatment for people with comorbid FXS and MDD.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

