Clinical Triaging in Cough Clinic Alleviates COVID-19 Overload in Emergency Department in India
- PMID: 33432307
- PMCID: PMC7788386
- DOI: 10.1007/s42399-020-00705-2
Clinical Triaging in Cough Clinic Alleviates COVID-19 Overload in Emergency Department in India
Abstract
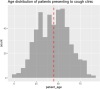
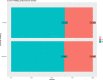
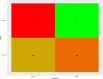
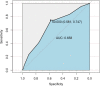
The importance of this study is the efficacy of "symptoms only" approach at a screening clinic for coronavirus disease 2019 (COVID-19) diagnosis in low- and middle-income countries (LMIC) setting. The objective of this study was to assess how efficiently primary care physicians at the screening clinic were able to predict whether a patient had COVID-19 or not, based on their symptom-based assessment alone. The current study is a cross-sectional retrospective observational study. This study was conducted at a single-center, tertiary care setting with a dedicated COVID-19 facility in a metropolitan city in eastern India. Participants are all suspected COVID-19 patients who presented themselves to this center during the outbreak from 1 August 2020 to 30 August 2020. Patients were referred to the Cough Clinic from the various outpatient departments of the hospital or from smaller satellite centers located in different parts of the city and other dependent geographical areas. The main outcome(s) and measure(s) is to study whether outcome of confirmatory test results can be predicted accurately by history taking alone. From 01 August 2020 to 30 Aug 2020, 511 patients with at least one symptom suggestive of COVID-19 reported to screening clinic. Out of these, 65.4% were males and 34.6% were females. Median age was 45 years with range being 01 to 92 years. Fever was seen in 70.4% while cough was present in 22% of cases. Overall positivity for SARS-CoV-2 during this period in this group was 54.21%. At 50% pre-test probability, the sensitivity of trained doctors working at the clinic, in predicting positive cases based on symptoms alone, was approximately 74.7%, and specificity for the same was 58.12%. The positive predictive value of the doctors' assessment was 67.87%, and the negative predictive value was 66.02%. Rapid triaging for confirmatory diagnosis of COVID-19 is feasible at screening clinic based on history taking alone by training of primary care physicians. This is particularly relevant in LMIC with scarce healthcare resources to overcome COVID-19 pandemic.
Keywords: COVID-19; History taking; Rapid triaging; Symptoms only approach.
© The Author(s), under exclusive licence to Springer Nature Switzerland AG part of Springer Nature 2021.
Conflict of interest statement
Conflict of InterestThe authors declare that they have no conflict of interest.
Figures

References
-
- Worldometers info Available from: https://www.worldometers.info/coronavirus/. Accessed 1 Oct 2020.
-
- Cdc.gov. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim.... Accessed 1 Oct 2020.
-
- World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Interim guidance, World Health Organization (WHO). 2020. https://www.who.int/publications-detail/clinicalmanagement-of-severe-acu.... Accessed 3 Oct 2020.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
