Molecular markers of telomere dysfunction and senescence are common findings in the usual interstitial pneumonia pattern of lung fibrosis
- PMID: 33432658
- PMCID: PMC8195814
- DOI: 10.1111/his.14334
Molecular markers of telomere dysfunction and senescence are common findings in the usual interstitial pneumonia pattern of lung fibrosis
Abstract
Aims: Idiopathic pulmonary fibrosis (IPF) is a genetically mediated, age-associated, progressive form of pulmonary fibrosis characterised pathologically by a usual interstitial pneumonia (UIP) pattern of fibrosis. The UIP pattern is also found in pulmonary fibrosis attributable to clinical diagnoses other than IPF (non-IPF UIP), whose clinical course is similarly poor, suggesting common molecular drivers. This study investigates whether IPF and non-IPF UIP lungs similarly express markers of telomere dysfunction and senescence.
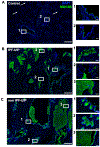
Methods and results: To test whether patients with IPF and non-IPF UIP share molecular drivers, lung tissues from 169 IPF patients and 57 non-IPF UIP patients were histopathologically and molecularly compared. Histopathological changes in both IPF and non-IPF UIP patients included temporal heterogeneity, microscopic honeycombing, fibroblast foci, and dense collagen fibrosis. Non-IPF UIP lungs were more likely to have lymphocytic infiltration, non-caseating granulomas, airway-centred inflammation, or small airways disease. Telomeres were shorter in alveolar type II (AECII) cells of both IPF and non-IPF UIP lungs than in those of age-similar, unused donor, controls. Levels of molecular markers of senescence (p16 and p21) were elevated in lysates of IPF and non-IPF UIP lungs. Immunostaining localised expression of these proteins to AECII cells. The mucin 5B (MUC5B) gene promoter variant minor allele frequency was similar between IPF and non-IPF UIP patients, and MUC5B expression was similar in IPF and non-IPF UIP lungs.
Conclusions: Molecular markers of telomere dysfunction and senescence are pathologically expressed in both IPF and non-IPF UIP lungs. These findings suggest that common molecular drivers may contribute to the pathogenesis of UIP-associated pulmonary fibrosis, regardless of the clinical diagnosis.
Keywords: alveolar type II cell; hypersensitivity pneumonitis; interstitial lung disease; pulmonary fibrosis; rheumatoid arthritis; scleroderma; senescence; telomere.
© 2021 John Wiley & Sons Ltd.
Figures




References
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–6. - PubMed
-
- Selman M, Lopez-Otin C, Pardo A. Age-driven developmental drift in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J. 2016;48(2):538–52. - PubMed
MeSH terms
Substances
Grants and funding
- KL2 TR000143/TR/NCATS NIH HHS/United States
- HL108794/HL/NHLBI NIH HHS/United States
- HL138131/HL/NHLBI NIH HHS/United States
- P30 NS048154/NS/NINDS NIH HHS/United States
- K23 HL138131/HL/NHLBI NIH HHS/United States
- UCSF-CTI KL2TR000143/TR/NCATS NIH HHS/United States
- P01 HL108794/HL/NHLBI NIH HHS/United States
- T32 HL007085/HL/NHLBI NIH HHS/United States
- UG3 HL151865/HL/NHLBI NIH HHS/United States
- R01 HL149836/HL/NHLBI NIH HHS/United States
- HL139897/HL/NHLBI NIH HHS/United States
- UH3 HL151865/HL/NHLBI NIH HHS/United States
- R01 NS086839/NS/NINDS NIH HHS/United States
- R01 HL139897/HL/NHLBI NIH HHS/United States
- I01 BX005295/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

