Population genomics in the arboviral vector Aedes aegypti reveals the genomic architecture and evolution of endogenous viral elements
- PMID: 33432714
- PMCID: PMC8048955
- DOI: 10.1111/mec.15798
Population genomics in the arboviral vector Aedes aegypti reveals the genomic architecture and evolution of endogenous viral elements
Abstract
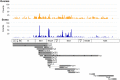
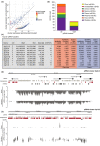
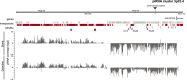
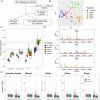
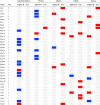
Horizontal gene transfer from viruses to eukaryotic cells is a pervasive phenomenon. Somatic viral integrations are linked to persistent viral infection whereas integrations into germline cells are maintained in host genomes by vertical transmission and may be co-opted for host functions. In the arboviral vector Aedes aegypti, an endogenous viral element from a nonretroviral RNA virus (nrEVE) was shown to produce PIWI-interacting RNAs (piRNAs) to limit infection with a cognate virus. Thus, nrEVEs may constitute a heritable, sequence-specific mechanism for antiviral immunity, analogous to piRNA-mediated silencing of transposable elements. Here, we combine population genomics and evolutionary approaches to analyse the genomic architecture of nrEVEs in A. aegypti. We conducted a genome-wide screen for adaptive nrEVEs and searched for novel population-specific nrEVEs in the genomes of 80 individual wild-caught mosquitoes from five geographical populations. We show a dynamic landscape of nrEVEs in mosquito genomes and identified five novel nrEVEs derived from two currently circulating viruses, providing evidence of the environmental-dependent modification of a piRNA cluster. Overall, our results show that virus endogenization events are complex with only a few nrEVEs contributing to adaptive evolution in A. aegypti.
Keywords: Aedes aegypti; endogenous viral elements; mosquito genomes; piRNA cluster.
© 2021 The Authors. Molecular Ecology published by John Wiley & Sons Ltd.
Figures


References
-
- Arensburger, P. , Hice, R. H. , Wright, J. A. , Craig, N. L. , & Atkinson, P. W. (2011). The mosquito Aedes aegypti has a large genome size and high transposable element load but contains a low proportion of transposon‐specific piRNAs. BMC Genomics, 12, 10.1186/1471-2164-12-606. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

