Risk and Risk Score Performance of Hepatocellular Carcinoma Development in Patients With Hepatitis B Surface Antigen Seroclearance
- PMID: 33433118
- PMCID: PMC7803670
- DOI: 10.14309/ctg.0000000000000290
Risk and Risk Score Performance of Hepatocellular Carcinoma Development in Patients With Hepatitis B Surface Antigen Seroclearance
Abstract
Introduction: Hepatocellular carcinoma (HCC) can develop among chronic hepatitis B patients after hepatitis B surface antigen (HBsAg) seroclearance. However, whether HCC risk after HBsAg seroclearance differs between antiviral therapy (AVT)-induced or spontaneous seroclearance cases and ways to identify at-risk populations remain unclear.
Methods: A retrospective cohort of 1,200 adult chronic hepatitis B patients who achieved HBsAg seroclearance (median age: 56 years; 824 men; 165 with cirrhosis; 216 AVT-induced cases) were analyzed. The risk of HCC after HBsAg seroclearance and the performance of 6 HCC prediction models were assessed.
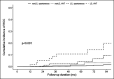
Results: During a median of 4.8 years of follow-up (range: 0.5-17.8 years), HCC developed in 23 patients (1.9%). The HCC incidence rate was higher in the AVT-induced cases than that in the spontaneous cases (3.9% vs 0.9% at 5 years). AVT and cirrhosis were independent factors associated with HCC, with HCC incidence rates of 0.5%, 1.2%, 4.0%, and 10.5% at 5 years for spontaneous/no-cirrhosis, AVT-induced/no-cirrhosis, spontaneous/cirrhosis, and AVT-induced/cirrhosis patients, respectively. Among the 6 predictive HCC models tested, Chinese University-HCC score (0.82) showed the highest C-statistics, which was followed by guide with age, gender, HBV DNA, core promoter mutations and cirrhosis (0.81).
Discussion: AVT-induced HBsAg seroclearance was associated with higher HCC risk, especially for patients with cirrhosis, indicating that they need careful monitoring for HCC risk. The HCC risk models were able to stratify the HCC risk in patients with HBsAg seroclearance.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

Similar articles
-
Risk of Hepatocellular Carcinoma Decreases After Antiviral Therapy-Induced HBsAg Seroclearance.J Gastroenterol Hepatol. 2025 Jul;40(7):1675-1685. doi: 10.1111/jgh.16973. Epub 2025 Apr 24. J Gastroenterol Hepatol. 2025. PMID: 40273951
-
Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance.J Hepatol. 2015 May;62(5):1092-9. doi: 10.1016/j.jhep.2014.11.031. Epub 2014 Nov 28. J Hepatol. 2015. PMID: 25445399
-
Long-term outcomes and predictive scores for hepatocellular carcinoma and hepatitis B surface antigen seroclearance after hepatitis B e-antigen seroclearance.Hepatology. 2018 Aug;68(2):462-472. doi: 10.1002/hep.29874. Epub 2018 Jun 6. Hepatology. 2018. PMID: 29534307
-
Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance.J Viral Hepat. 2018 Sep;25(9):1026-1037. doi: 10.1111/jvh.12905. Epub 2018 May 2. J Viral Hepat. 2018. PMID: 29624821
-
Systematic review with meta-analysis: development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance.Aliment Pharmacol Ther. 2016 Jun;43(12):1253-61. doi: 10.1111/apt.13634. Epub 2016 Apr 27. Aliment Pharmacol Ther. 2016. PMID: 27117732
Cited by
-
An Ideal Hallmark Closest to Complete Cure of Chronic Hepatitis B Patients: High-sensitivity Quantitative HBsAg Loss.J Clin Transl Hepatol. 2023 Feb 28;11(1):197-206. doi: 10.14218/JCTH.2022.00289. Epub 2022 Aug 22. J Clin Transl Hepatol. 2023. PMID: 36406318 Free PMC article. Review.
-
KASL clinical practice guidelines for management of chronic hepatitis B.Clin Mol Hepatol. 2022 Apr;28(2):276-331. doi: 10.3350/cmh.2022.0084. Epub 2022 Apr 1. Clin Mol Hepatol. 2022. PMID: 35430783 Free PMC article. No abstract available.
-
Novel Biomarkers of Hepatitis B Virus and Their Use in Chronic Hepatitis B Patient Management.Viruses. 2021 May 21;13(6):951. doi: 10.3390/v13060951. Viruses. 2021. PMID: 34064049 Free PMC article. Review.
-
Surveillance Following Hepatitis B Surface Antigen Loss: An Issue Requiring Attention.Pathogens. 2024 Dec 27;14(1):8. doi: 10.3390/pathogens14010008. Pathogens. 2024. PMID: 39860969 Free PMC article. Review.
-
Prevalence of hepatitis B virus infection and its associated factors among students in N'Djamena, Chad.PLoS One. 2024 Apr 18;19(4):e0273589. doi: 10.1371/journal.pone.0273589. eCollection 2024. PLoS One. 2024. PMID: 38635501 Free PMC article.
References
-
- Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529–38. - PubMed
-
- European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

