Prototypical Clinical Trial Registry Based on Fast Healthcare Interoperability Resources (FHIR): Design and Implementation Study
- PMID: 33433393
- PMCID: PMC7837997
- DOI: 10.2196/20470
Prototypical Clinical Trial Registry Based on Fast Healthcare Interoperability Resources (FHIR): Design and Implementation Study
Abstract
Background: Clinical trial registries increase transparency in medical research by making information and results of planned, ongoing, and completed studies publicly available. However, the registration of clinical trials remains a time-consuming manual task complicated by the fact that the same studies often need to be registered in different registries with different data entry requirements and interfaces.
Objective: This study investigates how Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR) may be used as a standardized format for exchanging and storing clinical trial records.
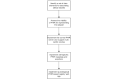
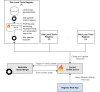
Methods: We designed and prototypically implemented an open-source central trial registry containing records from university hospitals, which are automatically exported and updated by local study management systems.
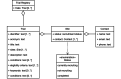
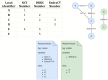
Results: We provided an architecture and implementation of a multisite clinical trials registry based on HL7 FHIR as a data storage and exchange format.
Conclusions: The results show that FHIR resources establish a harmonized view of study information from heterogeneous sources by enabling automated data exchange between trial centers and central study registries.
Keywords: HL7 FHIR; clinical trials; data sharing; health information interoperability; trials registry.
©Christian Gulden, Romina Blasini, Azadeh Nassirian, Alexandra Stein, Fatma Betül Altun, Melanie Kirchner, Hans-Ulrich Prokosch, Martin Boeker. Originally published in JMIR Medical Informatics (http://medinform.jmir.org), 12.01.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Baudard M, Yavchitz A, Ravaud P, Perrodeau E, Boutron I. Impact of searching clinical trial registries in systematic reviews of pharmaceutical treatments: methodological systematic review and reanalysis of meta-analyses. BMJ. 2017 Feb 17;356:j448. doi: 10.1136/bmj.j448. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=28213479 - DOI - PMC - PubMed
-
- Adam GP, Springs S, Trikalinos T, Williams JW, Eaton JL, Von Isenburg M, Gierisch JM, Wilson LM, Robinson KA, Viswanathan M, Middleton JC, Forman-Hoffman VL, Berliner E, Kaplan RM. Does information from ClinicalTrials.gov increase transparency and reduce bias? Results from a five-report case series. Syst Rev. 2018 Apr 16;7(1):59. doi: 10.1186/s13643-018-0726-5. https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s136... - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

