P2X7 receptor activation aggravates NADPH oxidase 2-induced oxidative stress after intracerebral hemorrhage
- PMID: 33433488
- PMCID: PMC8323669
- DOI: 10.4103/1673-5374.303036
P2X7 receptor activation aggravates NADPH oxidase 2-induced oxidative stress after intracerebral hemorrhage
Abstract
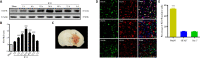
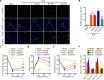
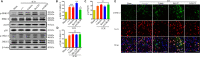
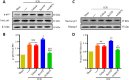
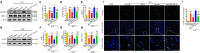
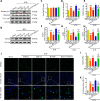
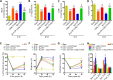
Oxidative stress is a crucial pathological process that contributes to secondary injury following intracerebral hemorrhage. P2X7 receptor (P2X7R), which is activated by the abnormal accumulation of extracellular ATP, plays an important role in the regulation of oxidative stress in the central nervous system, although the effects of activated P2X7R-associated oxidative stress after intracerebral hemorrhage remain unclear. Mouse models of intracerebral hemorrhage were established through the stereotactic injection of 0.075 U VII collagenase into the right basal ganglia. The results revealed that P2X7R expression peaked 24 hours after intracerebral hemorrhage, and P2X7R expressed primarily in neurons. The inhibition of P2X7R, using A438079 (100 mg/kg, intraperitoneal), reduced nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2) expression and malondialdehyde generation, increased superoxide dismutase and glutathione/oxidized glutathione levels, and alleviated neurological damage, brain edema, and apoptosis after intracellular hemorrhage. The P2X7R inhibitor A438079 (100 mg/kg, intraperitoneal injection) inhibited the activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and nuclear factor kappa-B (NF-κB) after intracerebral hemorrhage. Blocking ERK1/2 activation, using the ERK1/2 inhibitor U0126 (2 µg, intraventricular injection), reduced the level of NOX2-mediated oxidative stress induced by P2X7R activation after intracellular hemorrhage. Similarly, the inhibition of NF-κB, using the NF-κB inhibitor JSH-23 (3.5 µg, intraventricular), reduced the level of NOX2-mediated oxidative stress induced by P2X7R activation. Finally, GSK2795039 (100 mg/kg, intraperitoneal), a NOX2 antagonist, attenuated P2X7R-mediated oxidative stress, neurological damage, and brain edema after intracerebral hemorrhage. The results indicated that P2X7R activation aggravated NOX2-induced oxidative stress through the activation of the ERK1/2 and NF-κB pathways following intracerebral hemorrhage in mice. The present study was approved by the Ethics Committee of Huazhong University of Science and Technology, China (approval No. TJ-A20160805) on August 26, 2016.
Keywords: brain; central nervous system; factor; inflammation; injury; pathways; repair; stroke.
Conflict of interest statement
None
Figures

References
-
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. - PubMed
-
- Apolloni S, Fabbrizio P, Parisi C, Amadio S, Volonté C. Clemastine confers neuroprotection and induces an anti-inflammatory phenotype in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Mol Neurobiol. 2016;53:518–531. - PubMed
-
- Apolloni S, Parisi C, Pesaresi MG, Rossi S, Carrì MT, Cozzolino M, Volonté C, D’Ambrosi N. The NADPH oxidase pathway is dysregulated by the P2X7 receptor in the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J Immunol. 2013;190:5187–5195. - PubMed
-
- Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66:638–675. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

