Effect of Exposure to e-Cigarettes With Salt vs Free-Base Nicotine on the Appeal and Sensory Experience of Vaping: A Randomized Clinical Trial
- PMID: 33433597
- PMCID: PMC7804919
- DOI: 10.1001/jamanetworkopen.2020.32757
Effect of Exposure to e-Cigarettes With Salt vs Free-Base Nicotine on the Appeal and Sensory Experience of Vaping: A Randomized Clinical Trial
Abstract
Importance: Alkaline free-base nicotine is bitter and a respiratory irritant. High-nicotine electronic cigarette (e-cigarette) products contain acid additives that change nicotine from a free-base to a protonated salt chemical form, which could improve the sensory experience of vaping, particularly among never smokers unaccustomed to inhaling free-base nicotine.
Objective: To determine whether exposure to e-cigarettes with salt vs free-base nicotine formulations improves the appeal and sensory experience of vaping e-cigarettes and whether nicotine formulation effects differ by e-cigarette flavor and ever combustible cigarette smoking status.
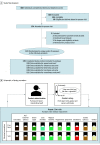
Design, setting, and participants: Single-visit double-blind within-participant randomized clinical trial was conducted in an academic medical center outpatient clinical research facility in Southern California. Participants were 119 individuals with past 30-day e-cigarette or combustible cigarette use aged 21 years or older recruited from November 2019 to March 2020.
Interventions: Participants self-administered standardized puffs of each 10 differently flavored e-cigarette solutions using a pod-style device. Each flavor was administered in salt (benzoic acid added) and free-base (no benzoic acid) nicotine formulations with commensurate nicotine concentrations (mean, 23.6 mg/mL). The 20 solutions were administered in randomly assigned sequences. Immediately after puffing each solution, participants rated appeal and sensory attributes.
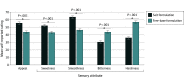
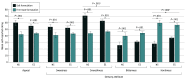
Main outcomes and measures: Self-reported appeal (mean of like, dislike [reverse-scored], and willingness to use again ratings) and 4 sensory attributes (sweetness, smoothness, bitterness, and harshness; analyzed individually) on visual analog scales with not at all and extremely anchors (range, 0-100).
Results: Of the 119 participants; 39 (32.8%) were female. The mean (SD) age was 42.1 (14.4) years; 105 (88.2%) were ever combustible cigarette smokers, and 66 (55.5%) were current e-cigarette users. Salt vs free-base nicotine formulations produced higher ratings of appeal (salt vs free-base mean difference effect estimate: b = 12.0; 95% CI, 9.9-14.1; P < .001), sweetness (b = 9.3; 95% CI, 7.1-11.4; P < .001), and smoothness (b = 17.4; 95% CI, 15.2-19.6; P < .001) and lower ratings of bitterness (b = -13.3; 95% CI, -15.4 to -11.2; P < .001) and harshness (b = -21.0; 95% CI, -23.2 to -18.7; P < .001). Nicotine formulation effects largely generalized across different flavors and the smoothness-enhancing and harshness-reducing effects of nicotine salt were stronger in never vs ever cigarette smokers.
Conclusions and relevance: In this randomized clinical trial of adult current nicotine or tobacco product users, controlled exposure to e-cigarette puffs with salt vs free-base nicotine formulations appeared to increase product appeal and improve the sensory experience of vaping, particularly among never smokers. Regulatory policies limiting acid additives in e-cigarettes might reduce the appeal of high-nicotine e-cigarettes among populations deterred from vaping e-cigarettes that emit harsh aerosol.
Trial registration: ClinicalTrials.gov Identifier: NCT04399031.
Conflict of interest statement
Figures

Similar articles
-
Dose-response effects of two nicotine salt formulations on electronic cigarette appeal and sensory attributes.Tob Control. 2023 Jan 2:tobaccocontrol-2022-057553. doi: 10.1136/tc-2022-057553. Online ahead of print. Tob Control. 2023. PMID: 36593119 Free PMC article.
-
Effects of 'Ice' flavoured e-cigarettes with synthetic cooling agent WS-23 or menthol on user-reported appeal and sensory attributes.Tob Control. 2025 Apr 1;34(2):175-182. doi: 10.1136/tc-2023-058125. Tob Control. 2025. PMID: 37940405 Free PMC article. Clinical Trial.
-
E-Cigarette Nicotine Delivery Among Young Adults by Nicotine Form, Concentration, and Flavor: A Crossover Randomized Clinical Trial.JAMA Netw Open. 2024 Aug 1;7(8):e2426702. doi: 10.1001/jamanetworkopen.2024.26702. JAMA Netw Open. 2024. PMID: 39120901 Free PMC article. Clinical Trial.
-
The Role of Nicotine and Flavor in the Abuse Potential and Appeal of Electronic Cigarettes for Adult Current and Former Cigarette and Electronic Cigarette Users: A Systematic Review.Nicotine Tob Res. 2022 Aug 6;24(9):1332-1343. doi: 10.1093/ntr/ntac073. Nicotine Tob Res. 2022. PMID: 35305014 Free PMC article.
-
Nicotine delivery and cigarette equivalents from vaping a JUULpod.Tob Control. 2022 Aug;31(e1):e88-e93. doi: 10.1136/tobaccocontrol-2020-056367. Epub 2021 Mar 24. Tob Control. 2022. PMID: 33762429 Free PMC article. Review.
Cited by
-
Electronic Nicotine Delivery Systems (ENDS), Marginalized Populations, and Tobacco Regulatory Policies.J Lung Health Dis. 2023;7(2):1-8. doi: 10.29245/2689-999x/2023/2.1183. J Lung Health Dis. 2023. PMID: 38235500 Free PMC article. No abstract available.
-
Usage Pattern and Nicotine Delivery during Ad Libitum Consumption of Pod E-Cigarettes and Heated Tobacco Products.Toxics. 2023 May 5;11(5):434. doi: 10.3390/toxics11050434. Toxics. 2023. PMID: 37235249 Free PMC article.
-
Decisions of the FDA on premarket tobacco product applications: Changes in the number of unique devices and liquids used by US adults who frequently use electronic nicotine delivery systems, 2020-2023.Tob Induc Dis. 2024 Mar 13;22. doi: 10.18332/tid/184240. eCollection 2024. Tob Induc Dis. 2024. PMID: 38482508 Free PMC article.
-
Effects of a fruit-ice combination flavor on appeal and sensory experience of vaping and moderation by preexisting e-cigarette flavor preference.Exp Clin Psychopharmacol. 2024 Dec;32(6):737-744. doi: 10.1037/pha0000731. Epub 2024 Aug 29. Exp Clin Psychopharmacol. 2024. PMID: 39207401 Clinical Trial.
-
Changing patterns of cigarette and ENDS transitions in the USA: a multistate transition analysis of adults in the PATH Study in 2017-2019 vs 2019-2021.Tob Control. 2024 Aug 22:tc-2023-058453. doi: 10.1136/tc-2023-058453. Online ahead of print. Tob Control. 2024. PMID: 39174323
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

