Kratom Alkaloids, Natural and Semi-Synthetic, Show Less Physical Dependence and Ameliorate Opioid Withdrawal
- PMID: 33433723
- PMCID: PMC8164968
- DOI: 10.1007/s10571-020-01034-7
Kratom Alkaloids, Natural and Semi-Synthetic, Show Less Physical Dependence and Ameliorate Opioid Withdrawal
Abstract
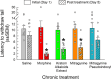
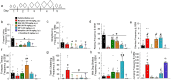
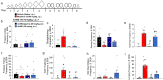
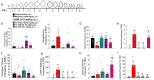
Chronic administration of opioids produces physical dependence and opioid-induced hyperalgesia. Users claim the Thai traditional tea "kratom" and component alkaloid mitragynine ameliorate opioid withdrawal without increased sensitivity to pain. Testing these claims, we assessed the combined kratom alkaloid extract (KAE) and two individual alkaloids, mitragynine (MG) and the analog mitragynine pseudoindoxyl (MP), evaluating their ability to produce physical dependence and induce hyperalgesia after chronic administration, and as treatments for withdrawal in morphine-dependent subjects. C57BL/6J mice (n = 10/drug) were administered repeated saline, or graded, escalating doses of morphine (intraperitoneal; i.p.), kratom alkaloid extract (orally, p.o.), mitragynine (p.o.), or MP (subcutaneously, s.c.) for 5 days. Mice treated chronically with morphine, KAE, or mitragynine demonstrated significant drug-induced hyperalgesia by day 5 in a 48 °C warm-water tail-withdrawal test. Mice were then administered naloxone (10 mg/kg, s.c.) and tested for opioid withdrawal signs. Kratom alkaloid extract and the two individual alkaloids demonstrated significantly fewer naloxone-precipitated withdrawal signs than morphine-treated mice. Additional C57BL/6J mice made physically dependent on morphine were then used to test the therapeutic potential of combined KAE, mitragynine, or MP given twice daily over the next 3 days at either a fixed dose or in graded, tapering descending doses. When administered naloxone, mice treated with KAE, mitragynine, or MP under either regimen demonstrated significantly fewer signs of precipitated withdrawal than control mice that continued to receive morphine. In conclusion, while retaining some liabilities, kratom, mitragynine, and mitragynine pseudoindoxyl produced significantly less physical dependence and ameliorated precipitated withdrawal in morphine-dependent animals, suggesting some clinical value.
Keywords: Kratom; Mitragynine; Opioid; Physical dependence; Withdrawal.
Conflict of interest statement
The authors have no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

