Degenerative lamellar macular holes: tractional development and morphological alterations
- PMID: 33433772
- PMCID: PMC8035119
- DOI: 10.1007/s10792-020-01674-0
Degenerative lamellar macular holes: tractional development and morphological alterations
Abstract
Purpose: The development of degenerative lamellar macular holes (DLH) is largely unclear. This study was aimed at documenting with spectral-domain optical coherence tomography the tractional development and morphological alterations of DLH.
Methods: A retrospective case series of 44 eyes of 44 patients is described.
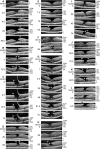
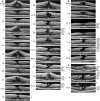
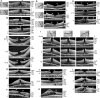
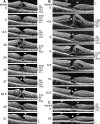
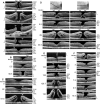
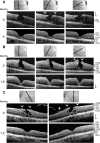
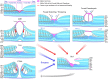
Results: The development of DLH is preceded for months or years by tractional deformations of the fovea due to the action of contractile epiretinal membranes (ERM) and/or the partially detached posterior hyaloid, or by cystoid macular edema (CME). DLH may develop after a tractional stretching and thickening of the foveal center, from a foveal pseudocyst, after a detachment of the foveola from the retinal pigment epithelium, a disruption of the foveal structure due to CME, and after surgical treatment of tractional lamellar or full-thickness macular holes (FTMH). The foveal configuration of a DLH can be spontaneously reestablished after short transient episodes of CME and a small FTMH. A DLH can evolve to a FTMH by traction of an ERM. Surgical treatment of a DLH may result in an irregular regeneration of the foveal center without photoreceptors.
Conclusions: Tractional forces play an important role in the development of DLH and in the further evolution to FTMH. It is suggested that a DLH is the result of a retinal wound repair process after a tractional disruption of the Müller cell cone and a degeneration of Henle fibers, to prevent a further increase in the degenerative cavitations.
Keywords: Fovea; Full-thickness macular hole; Lamellar macular hole; Müller cell cone; Müller glia.
Figures
References
-
- Yamada E. Some structural features of the fovea centralis in the human retina. Arch Ophthalmol. 1969;82:151–159. - PubMed
-
- Gass JDM. Müller cell cone, an overlooked part of the anatomy of the fovea centralis: Hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch Ophthalmol. 1999;117:821–823. - PubMed
-
- Syrbe S, Kuhrt H, Gärtner U, Habermann G, Wiedemann P, Bringmann A, Reichenbach A. Müller glial cells of the primate foveola: An electron microscopical study. Exp Eye Res. 2018;167:110–117. - PubMed
-
- Bringmann A, Syrbe S, Görner K, Kacza J, Francke M, Wiedemann P, Reichenbach A. The primate fovea: structure, function and development. Prog Retin Eye Res. 2018;66:49–84. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

