High-Sensitivity Glycan Profiling of Blood-Derived Immunoglobulin G, Plasma, and Extracellular Vesicle Isolates with Capillary Zone Electrophoresis-Mass Spectrometry
- PMID: 33433994
- PMCID: PMC7987205
- DOI: 10.1021/acs.analchem.0c03102
High-Sensitivity Glycan Profiling of Blood-Derived Immunoglobulin G, Plasma, and Extracellular Vesicle Isolates with Capillary Zone Electrophoresis-Mass Spectrometry
Abstract
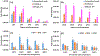
We developed a highly sensitive method for profiling of N-glycans released from proteins based on capillary zone electrophoresis coupled to electrospray ionization mass spectrometry (CZE-ESI-MS) and applied the technique to glycan analysis of plasma and blood-derived isolates. The combination of dopant-enriched nitrogen (DEN)-gas introduced into the nanoelectrospray microenvironment with optimized ionization, desolvation, and CZE-MS conditions improved the detection sensitivity up to ∼100-fold, as directly compared to the conventional mode of instrument operation through peak intensity measurements. Analyses without supplemental pressure increased the resolution ∼7-fold in the separation of closely related and isobaric glycans. The developed method was evaluated for qualitative and quantitative glycan profiling of three types of blood isolates: plasma, total serum immunoglobulin G (IgG), and total plasma extracellular vesicles (EVs). The comparative glycan analysis of IgG and EV isolates and total plasma was conducted for the first time and resulted in detection of >200, >400, and >500 N-glycans for injected sample amounts equivalent to <500 nL of blood. Structural CZE-MS2 analysis resulted in the identification of highly diverse glycans, assignment of α-2,6-linked sialic acids, and differentiation of positional isomers. Unmatched depth of N-glycan profiling was achieved compared to previously reported methods for the analysis of minute amounts of similar complexity blood isolates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

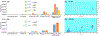



Similar articles
-
Highly-sensitive label-free deep profiling of N-glycans released from biomedically-relevant samples.Nat Commun. 2023 Mar 23;14(1):1618. doi: 10.1038/s41467-023-37365-4. Nat Commun. 2023. PMID: 36959283 Free PMC article.
-
Glycoprotein characterization combining intact protein and glycan analysis by capillary electrophoresis-electrospray ionization-mass spectrometry.Anal Chem. 2006 Aug 1;78(15):5384-93. doi: 10.1021/ac060376g. Anal Chem. 2006. PMID: 16878873
-
Simple capillary electrophoresis-mass spectrometry method for complex glycan analysis using a flow-through microvial interface.Anal Chem. 2014 Jul 1;86(13):6479-86. doi: 10.1021/ac5010212. Epub 2014 Jun 13. Anal Chem. 2014. PMID: 24873509
-
Chromatography and its hyphenation to mass spectrometry for extracellular vesicle analysis.J Chromatogr A. 2016 Mar 25;1439:26-41. doi: 10.1016/j.chroma.2016.01.017. Epub 2016 Jan 11. J Chromatogr A. 2016. PMID: 26830636 Review.
-
Capillary Electrophoresis Separations of Glycans.Chem Rev. 2018 Sep 12;118(17):7867-7885. doi: 10.1021/acs.chemrev.7b00669. Epub 2018 Mar 12. Chem Rev. 2018. PMID: 29528644 Free PMC article. Review.
Cited by
-
[Annual review of capillary electrophoresis technology in 2021].Se Pu. 2022 Jul;40(7):591-599. doi: 10.3724/SP.J.1123.2022.03040. Se Pu. 2022. PMID: 35791597 Free PMC article. Review. Chinese.
-
Immunoaffinity Capillary Electrophoresis in the Era of Proteoforms, Liquid Biopsy and Preventive Medicine: A Potential Impact in the Diagnosis and Monitoring of Disease Progression.Biomolecules. 2021 Oct 1;11(10):1443. doi: 10.3390/biom11101443. Biomolecules. 2021. PMID: 34680076 Free PMC article. Review.
-
Improved Data Acquisition Settings on Q Exactive HF-X and Fusion Lumos Tribrid Orbitrap-Based Mass Spectrometers for Proteomic Analysis of Limited Samples.J Proteome Res. 2024 Jun 7;23(6):2230-2240. doi: 10.1021/acs.jproteome.4c00181. Epub 2024 May 1. J Proteome Res. 2024. PMID: 38690845 Free PMC article.
-
Capillary Electrophoresis Coupled to Electrospray Ionization Tandem Mass Spectrometry for Ultra-Sensitive Proteomic Analysis of Limited Samples.Anal Chem. 2022 Jan 18;94(2):704-713. doi: 10.1021/acs.analchem.1c02929. Epub 2022 Jan 4. Anal Chem. 2022. PMID: 34983182 Free PMC article.
-
Glycan Node Analysis Detects Varying Glycosaminoglycan Levels in Melanoma-Derived Extracellular Vesicles.Int J Mol Sci. 2023 May 9;24(10):8506. doi: 10.3390/ijms24108506. Int J Mol Sci. 2023. PMID: 37239852 Free PMC article.
References
-
- Varki A; Kannagi R; Toole B; Stanley P Glycosylation Changes in Cancer. In Essentials of Glycobiology, 3rd ed.; Varki A Ed., Cold Spring Harbor: NY, 2017; Chapter 47.
-
- Lauc G; Pezer M; Rudan I; Campbell H Biochim. Biophys. Acta 2016, 1860, 1574–1582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

