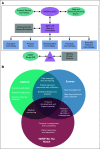
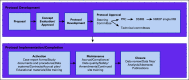
Building a Fit for Purpose Clinical Trials Infrastructure to Accelerate the Assessment of Novel Hematopoietic Cell Transplantation Strategies and Cellular Immunotherapies
- PMID: 33434065
- PMCID: PMC8443822
- DOI: 10.1200/JCO.20.01623
Building a Fit for Purpose Clinical Trials Infrastructure to Accelerate the Assessment of Novel Hematopoietic Cell Transplantation Strategies and Cellular Immunotherapies
Figures
References
-
- Markham MJ, Wachter K, Agarwal N, et al. :Clinical cancer advances 2020: Annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 38:1081, 2020 - PubMed
-
- Siegel RL, Miller KD, Jemal A:Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 - PubMed
-
- Weiden PL, Flournoy N, Thomas ED, et al. :Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 300:1068-1073, 1979 - PubMed
Publication types
MeSH terms
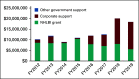
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

