Tetracyclines improve experimental lymphatic filariasis pathology by disrupting interleukin-4 receptor-mediated lymphangiogenesis
- PMID: 33434186
- PMCID: PMC7919730
- DOI: 10.1172/JCI140853
Tetracyclines improve experimental lymphatic filariasis pathology by disrupting interleukin-4 receptor-mediated lymphangiogenesis
Abstract
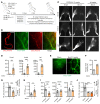
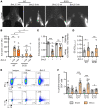
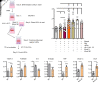
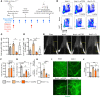
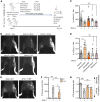
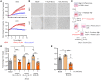
Lymphatic filariasis is the major global cause of nonhereditary lymphedema. We demonstrate that the filarial nematode Brugia malayi induced lymphatic remodeling and impaired lymphatic drainage following parasitism of limb lymphatics in a mouse model. Lymphatic insufficiency was associated with elevated circulating lymphangiogenic mediators, including vascular endothelial growth factor C. Lymphatic insufficiency was dependent on type 2 adaptive immunity, the interleukin-4 receptor, and recruitment of C-C chemokine receptor-2-positive monocytes and alternatively activated macrophages with a prolymphangiogenic phenotype. Oral treatments with second-generation tetracyclines improved lymphatic function, while other classes of antibiotic had no significant effect. Second-generation tetracyclines directly targeted lymphatic endothelial cell proliferation and modified type 2 prolymphangiogenic macrophage development. Doxycycline treatment impeded monocyte recruitment, inhibited polarization of alternatively activated macrophages, and suppressed T cell adaptive immune responses following infection. Our results determine a mechanism of action for the antimorbidity effects of doxycycline in filariasis and support clinical evaluation of second-generation tetracyclines as affordable, safe therapeutics for lymphedemas of chronic inflammatory origin.
Keywords: Infectious disease; Inflammation; Lymph; Parasitology; Th2 response.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

