Choosing important health outcomes for comparative effectiveness research: 6th annual update to a systematic review of core outcome sets for research
- PMID: 33434219
- PMCID: PMC7802923
- DOI: 10.1371/journal.pone.0244878
Choosing important health outcomes for comparative effectiveness research: 6th annual update to a systematic review of core outcome sets for research
Abstract
Background: An annual update to a systematic review of core outcome sets (COS) for research ensures that the COMET database is up-to-date. The aims of this study were to: (i) identify COS that were published or indexed in 2019 and to describe the methodological approaches used in these studies; (ii) investigate whether children have been included as participants in published COS development studies, and which methods have been used to facilitate their participation; iii) update a previous exercise to identify COS relevant to the most burdensome global diseases and injuries.
Methods: MEDLINE and SCOPUS were searched to identify studies published or indexed between (and inclusive of) January 2019 and December 2019. Automated screening methods were used to rank the citations in order of relevance; the top 25% in ranked priority order were screened for eligibility. COS were assessed against each of the Core Outcome Set-STAndards for Development (COS-STAD). A search of the COMET database was undertaken to identify COS relevant to the 25 leading causes of disease burden.
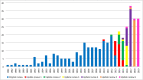
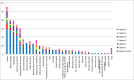
Results: Thirty-three studies, describing the development of 37 COS, were included in this update. These studies have been added to the COMET database, which now contains 370 published (1981-2019) COS studies for clinical research. Six (18%) of the 33 studies in this update were deemed to have met all of the minimum standards for COS development (range = 4 to 12 criteria, median = 9 criteria). Of the 370 COS studies published to date, 82 COS have been developed for paediatric health conditions and children would have been eligible to participate in 68/82 of these studies. Eleven of these 68 (16%) COS studies have included children as participants within the development process, most commonly through participation in Delphi surveys. Relevant COS were identified for 22/25 leading causes of global disease burden.
Conclusion: There has been a demonstrated increase in COS developed for both research and routine practice, and consistently high inclusion of patient participants. COS developed for paediatric conditions need to further incorporate the perspectives of children, alongside parents and other adults, and adopt research methods fit for this purpose. COS developers should consider the gaps identified in this update as priorities for COS development.
Conflict of interest statement
EG and PRW are members of the COMET Management Group. SLG and KMS have declared that no competing interests exist. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Gorst SL, Gargon E, Clarke M, Smith V, Williamson PR. Choosing Important Health Outcomes for Comparative Effectiveness Research: An Updated Review and Identification of Gaps. PLoS One. 2016;11(12):e0168403 Epub 2016/12/16. 10.1371/journal.pone.0168403 to support COMET and related work. EG is a member of the COMET Management Group and is the COMET Project Co-ordinator. This does not alter the authors’ adherence to PLOS ONE policies on sharing data and materials. - DOI - PMC - PubMed
-
- Davis K, Gorst SL, Harman N, Smith V, Gargon E, Altman DG, et al. Choosing important health outcomes for comparative effectiveness research: An updated systematic review and involvement of low and middle income countries. PLoS One. 2018;13(2):e0190695 Epub 2018/02/14. 10.1371/journal.pone.0190695 - DOI - PMC - PubMed
-
- Gargon E, Gorst SL, Harman NL, Smith V, Matvienko-Sikar K, Williamson PR. Choosing important health outcomes for comparative effectiveness research: 4th annual update to a systematic review of core outcome sets for research. PLoS One. 2018;13(12):e0209869 Epub 2018/12/29. 10.1371/journal.pone.0209869 competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous