Mast Cells Promote Nonalcoholic Fatty Liver Disease Phenotypes and Microvesicular Steatosis in Mice Fed a Western Diet
- PMID: 33434322
- PMCID: PMC9271361
- DOI: 10.1002/hep.31713
Mast Cells Promote Nonalcoholic Fatty Liver Disease Phenotypes and Microvesicular Steatosis in Mice Fed a Western Diet
Abstract
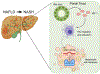
Background and aims: Nonalcoholic fatty liver disease (NAFLD) is simple steatosis but can develop into nonalcoholic steatohepatitis (NASH), characterized by liver inflammation, fibrosis, and microvesicular steatosis. Mast cells (MCs) infiltrate the liver during cholestasis and promote ductular reaction (DR), biliary senescence, and liver fibrosis. We aimed to determine the effects of MC depletion during NAFLD/NASH.
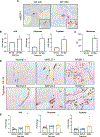
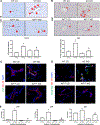
Approach and results: Wild-type (WT) and KitW-sh (MC-deficient) mice were fed a control diet (CD) or a Western diet (WD) for 16 weeks; select WT and KitW-sh WD mice received tail vein injections of MCs 2 times per week for 2 weeks prior to sacrifice. Human samples were collected from normal, NAFLD, or NASH mice. Cholangiocytes from WT WD mice and human NASH have increased insulin-like growth factor 1 expression that promotes MC migration/activation. Enhanced MC presence was noted in WT WD mice and human NASH, along with increased DR. WT WD mice had significantly increased steatosis, DR/biliary senescence, inflammation, liver fibrosis, and angiogenesis compared to WT CD mice, which was significantly reduced in KitW-sh WD mice. Loss of MCs prominently reduced microvesicular steatosis in zone 1 hepatocytes. MC injection promoted WD-induced biliary and liver damage and specifically up-regulated microvesicular steatosis in zone 1 hepatocytes. Aldehyde dehydrogenase 1 family, member A3 (ALDH1A3) expression is reduced in WT WD mice and human NASH but increased in KitW-sh WD mice. MicroRNA 144-3 prime (miR-144-3p) expression was increased in WT WD mice and human NASH but reduced in KitW-sh WD mice and was found to target ALDH1A3.
Conclusions: MCs promote WD-induced biliary and liver damage and may promote microvesicular steatosis development during NAFLD progression to NASH through miR-144-3p/ALDH1A3 signaling. Inhibition of MC activation may be a therapeutic option for NAFLD/NASH treatment.
© 2021 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures





References
-
- Popescu M, Popescu IA, Stanciu M, Cazacu SM, Ianosi NG, Comanescu MV, et al. Non-alcoholic fatty liver disease—clinical and histopathological aspects. Rom J Morphol Embryol 2016;57:1295–1302 - PubMed
-
- Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier M-H, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology 2002;35:1485–1493. - PubMed
-
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131. - PubMed
-
- Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther 1995;67:101–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

