Regulation of distinct caspase-8 functions in retinal ganglion cells and astroglia in experimental glaucoma
- PMID: 33434617
- PMCID: PMC10294121
- DOI: 10.1016/j.nbd.2021.105258
Regulation of distinct caspase-8 functions in retinal ganglion cells and astroglia in experimental glaucoma
Abstract
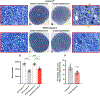
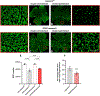
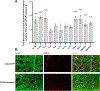
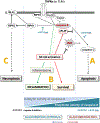
Retinal ganglion cells (RGCs) expanding from the retina to the brain are primary victims of neurodegeneration in glaucoma, a leading cause of blindness; however, the neighboring astroglia survive the glaucoma-related stress and promote neuroinflammation. In light of diverse functions of caspase-8 in apoptosis, cell survival, and inflammation, this study investigated the importance of caspase-8 in different fates of glaucomatous RGCs and astroglia using two experimental approaches in parallel. In the first approach, cell type-specific responses of RGCs and astroglia to a caspase-8 cleavage-inhibiting pharmacological treatment were studied in rat eyes with or without experimentally induced glaucoma. The second approach utilized an experimental model of glaucoma in mice in which astroglial caspase-8 was conditionally deleted by cre/lox. Findings of these experiments revealed cell type-specific distinct processes that regulate caspase-8 functions in experimental glaucoma, which are involved in inducing the apoptosis of RGCs and promoting the survival and inflammatory responses of astroglia. Deletion of caspase-8 in astroglia protected RGCs against glia-driven inflammatory injury, while the inhibition of caspase-8 cleavage inhibited apoptosis in RGCs themselves. Various caspase-8 functions impacting both RGC apoptosis and astroglia-driven neuroinflammation may suggest the multi-target potential of caspase-8 regulation to provide neuroprotection and immunomodulation in glaucoma.
Keywords: Astroglia; Caspase-8; Glaucoma; Neurodegeneration; Neuroinflammation.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



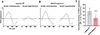

Similar articles
-
Multifactorial Pathogenic Processes of Retinal Ganglion Cell Degeneration in Glaucoma towards Multi-Target Strategies for Broader Treatment Effects.Cells. 2021 Jun 2;10(6):1372. doi: 10.3390/cells10061372. Cells. 2021. PMID: 34199494 Free PMC article. Review.
-
Transgenic inhibition of astroglial NF-κB restrains the neuroinflammatory and neurodegenerative outcomes of experimental mouse glaucoma.J Neuroinflammation. 2020 Aug 28;17(1):252. doi: 10.1186/s12974-020-01930-1. J Neuroinflammation. 2020. PMID: 32859212 Free PMC article.
-
cFLIP in the molecular regulation of astroglia-driven neuroinflammation in experimental glaucoma.J Neuroinflammation. 2024 Jun 1;21(1):145. doi: 10.1186/s12974-024-03141-4. J Neuroinflammation. 2024. PMID: 38824526 Free PMC article.
-
Role of hypoxia-inducible factor-1α in preconditioning-induced protection of retinal ganglion cells in glaucoma.Mol Vis. 2013 Nov 23;19:2360-72. eCollection 2013. Mol Vis. 2013. PMID: 24319330 Free PMC article.
-
Risk Factors for Retinal Ganglion Cell Distress in Glaucoma and Neuroprotective Potential Intervention.Int J Mol Sci. 2021 Jul 27;22(15):7994. doi: 10.3390/ijms22157994. Int J Mol Sci. 2021. PMID: 34360760 Free PMC article. Review.
Cited by
-
Multiplex protein analysis for the study of glaucoma.Expert Rev Proteomics. 2021 Oct;18(10):911-924. doi: 10.1080/14789450.2021.1996232. Epub 2021 Oct 29. Expert Rev Proteomics. 2021. PMID: 34672220 Free PMC article. Review.
-
Apoptosis in glaucoma: A new direction for the treatment of glaucoma (Review).Mol Med Rep. 2024 May;29(5):82. doi: 10.3892/mmr.2024.13207. Epub 2024 Mar 22. Mol Med Rep. 2024. PMID: 38516770 Free PMC article. Review.
-
Multifactorial Pathogenic Processes of Retinal Ganglion Cell Degeneration in Glaucoma towards Multi-Target Strategies for Broader Treatment Effects.Cells. 2021 Jun 2;10(6):1372. doi: 10.3390/cells10061372. Cells. 2021. PMID: 34199494 Free PMC article. Review.
-
Structural measurements and vessel density of spectral-domain optic coherence tomography in early, moderate, and severe primary angle-closure glaucoma.Int J Ophthalmol. 2023 Jul 18;16(7):1100-1109. doi: 10.18240/ijo.2023.07.15. eCollection 2023. Int J Ophthalmol. 2023. PMID: 37465514 Free PMC article.
-
Cistanche deserticola polysaccharides alleviate cognitive decline in aging model mice by restoring the gut microbiota-brain axis.Aging (Albany NY). 2021 Jun 3;13(11):15320-15335. doi: 10.18632/aging.203090. Epub 2021 Jun 3. Aging (Albany NY). 2021. PMID: 34081627 Free PMC article.
References
-
- Burguillos MA, Deierborg T, Kavanagh E, et al., 2011. Caspase signalling controls microglia activation and neurotoxicity. Nature. 472, 319–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

