Development of a quantitative one-step multiplex RT-qPCR assay for the detection of SARS-CoV-2 in a biological matrix
- PMID: 33434663
- PMCID: PMC7831874
- DOI: 10.1016/j.ijid.2021.01.001
Development of a quantitative one-step multiplex RT-qPCR assay for the detection of SARS-CoV-2 in a biological matrix
Abstract
Introduction: Coronavirus disease-2019 (COVID-19) is a disease caused by Severe Acute Respiratory Syndrome Virus 2 (SARS-CoV-2) that emerged in China in late 2019. The rapid viral spread has made the disease a public health emergency of worldwide concern. The gold standard for diagnosing SARS-CoV-2 is reverse transcription followed by qualitative real-time polymerase chain reaction (RT-qPCR); however, the role of viral load quantification has not been thoroughly investigated yet.
Objective: The aim of this study was to develop a high-precision quantitative one-step RT-qPCR reaction using the association of the viral target and the human target in the same reaction.
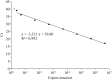
Methods: The assay standardization involved the absolute quantification method, with serial dilutions of a plasmid with the N gene in a biological matrix to build a standard curve.
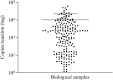
Results and discussion: The results demonstrated the possibility of quantifying as few as 2.5 copies/reaction and an analysis of 244 patients with known results selected by cross-section that revealed 100% agreement with a qualitative RT-qPCR assay registered by Anvisa. In this population, it was possible to quantify patients with between 2.59 and 3.5 × 107 copies per reaction and negative patients continued to indicate the same result.
Conclusion: This assay can be a useful tool for a proper patient management, because the level and duration of viral replication are important factors to assess the risk of transmission and to guide decisions regarding the isolation and release of patients; an accurate diagnosis is critical information, whereas the current COVID-19 pandemic represents the biggest current global health problem.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Real-time PCR-based SARS-CoV-2 detection.:https://preprints.scielo.org/index.php/scielo/prep.
-
- Borg I., Rohde G., Löseke S., Bittscheidt J., Schultze-Werninghaus G., Stephan V. Evaluation of a quantitative real-time PCR for the detection of respiratory syncytial virus in pulmonary diseases. Eur Respir J [Internet] 2003;21(6 June):944–951. doi: 10.1183/09031936.03.00088102. Available from: - DOI - PubMed
-
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Napoli R Di. 2020. Features, Evaluation and Treatment Coronavirus (COVID-19) [Internet]. Features, Evaluation and Treatment Coronavirus (COVID-19)https://www.ncbi.nlm.nih.gov/books/NBK554776/ [cited 2020 Jul 17]. Available from: - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

