A statistical framework for determination of minimal plasmid copy number required for transgene expression in mammalian cells
- PMID: 33434786
- PMCID: PMC7887061
- DOI: 10.1016/j.bioelechem.2020.107731
A statistical framework for determination of minimal plasmid copy number required for transgene expression in mammalian cells
Abstract
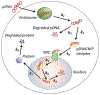
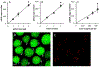
Plasmid DNA (pDNA) has been widely used for non-viral gene delivery. After pDNA molecules enter a mammalian cell, they may be trapped in subcellular structures or degraded by nucleases. Only a fraction of them can function as templates for transcription in the nucleus. Thus, an important question is, what is the minimal amount of pDNA molecules that need to be delivered into a cell for transgene expression? At present, it is technically a challenge to experimentally answer the question. To this end, we developed a statistical framework to establish the relationship between two experimentally quantifiable factors - average copy number of pDNA per cell among a group of cells after transfection and percent of the cells with transgene expression. The framework was applied to the analysis of electrotransfection under different experimental conditions in vitro. We experimentally varied the average copy number per cell and the electrotransfection efficiency through changes in extracellular pDNA dose, electric field strength, and pulse number. The experimental data could be explained or predicted quantitatively by the statistical framework. Based on the data and the framework, we could predict that the minimal number of pDNA molecules in the nucleus for transgene expression was on the order of 10. Although the prediction was dependent on the cell and experimental conditions used in the study, the framework may be generally applied to analysis of non-viral gene delivery.
Keywords: Copy number of DNA; Electroporation; Electrotransfection; Mathematical modeling; Non-viral gene delivery.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


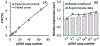

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

