O-GlcNAcylated peptides and proteins for structural and functional studies
- PMID: 33434850
- PMCID: PMC8222092
- DOI: 10.1016/j.sbi.2020.12.005
O-GlcNAcylated peptides and proteins for structural and functional studies
Abstract
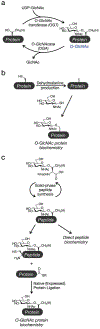
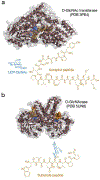
O-GlcNAcylation is an enzymatic post-translational modification occurring in hundreds of protein substrates. This modification occurs through the addition of the monosaccharide N-acetylglucosamine to serine and threonine residues on intracellular proteins in the cytosol, nucleus, and mitochondria. As a highly dynamic form of modification, changes in O-GlcNAc levels coincide with alterations in metabolic state, the presence of stressors, and cellular health. At the protein level, the consequences of the sugar modification can vary, thus necessitating biochemical investigations on protein-specific and site-specific effects. To this end, enzymatic and chemical methods to 'encode' the modification have been developed and the utilization of these synthetic glycopeptides and glycoproteins has since been instrumental in the discovery of the mechanisms by which O-GlcNAcylation can affect a diverse array of biological processes.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interests: None
Figures

Similar articles
-
MS-based proteomics for comprehensive investigation of protein O-GlcNAcylation.Mol Omics. 2021 Apr 19;17(2):186-196. doi: 10.1039/d1mo00025j. Mol Omics. 2021. PMID: 33687411 Review.
-
[Precise identification of O-linked β-N-acetylglucosamine peptides based on O-mesitylenesulfonylhydroxylamine elimination reaction].Se Pu. 2021 Nov;39(11):1182-1190. doi: 10.3724/SP.J.1123.2020.12024. Se Pu. 2021. PMID: 34677013 Free PMC article. Chinese.
-
The Sulfur-Linked Analogue of O-GlcNAc (S-GlcNAc) Is an Enzymatically Stable and Reasonable Structural Surrogate for O-GlcNAc at the Peptide and Protein Levels.Biochemistry. 2017 Jul 11;56(27):3507-3517. doi: 10.1021/acs.biochem.7b00268. Epub 2017 Jun 30. Biochemistry. 2017. PMID: 28627871 Free PMC article.
-
Identification of structural and functional O-linked N-acetylglucosamine-bearing proteins in Xenopus laevis oocyte.Mol Cell Proteomics. 2008 Nov;7(11):2229-45. doi: 10.1074/mcp.M700494-MCP200. Epub 2008 Jul 9. Mol Cell Proteomics. 2008. PMID: 18617508
-
Advances in Strategies and Tools Available for Interrogation of Protein O-GlcNAcylation.Chembiochem. 2021 Nov 3;22(21):3010-3026. doi: 10.1002/cbic.202100219. Epub 2021 Jun 22. Chembiochem. 2021. PMID: 34101962 Review.
Cited by
-
A sticky solution to protein-selective sugar installation.Cell Res. 2023 Jul;33(7):493-494. doi: 10.1038/s41422-023-00787-2. Cell Res. 2023. PMID: 36813880 Free PMC article. No abstract available.
-
Combining non-canonical amino acid mutagenesis and native chemical ligation for multiply modifying proteins: A case study of α-synuclein post-translational modifications.Methods. 2023 Oct;218:101-109. doi: 10.1016/j.ymeth.2023.08.002. Epub 2023 Aug 6. Methods. 2023. PMID: 37549799 Free PMC article.
-
O-GlcNAcylation of High Mobility Group Box 1 (HMGB1) Alters Its DNA Binding and DNA Damage Processing Activities.J Am Chem Soc. 2021 Oct 6;143(39):16030-16040. doi: 10.1021/jacs.1c06192. Epub 2021 Sep 21. J Am Chem Soc. 2021. PMID: 34546745 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

