Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis
- PMID: 33435236
- PMCID: PMC7827682
- DOI: 10.3390/cells10010120
Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis
Abstract
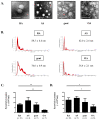
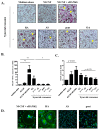
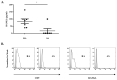
This study aimed to investigate the characteristics of exosomes isolated from synovial fluid and their role in osteoclast differentiation in different types of inflammatory arthritis. Exosomes isolated from synovial fluid of rheumatoid arthritis (RA), ankylosing spondylitis (AS), gout, and osteoarthritis (OA) patients were co-incubated with CD14+ mononuclear cells from healthy donors without macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-B ligand (RANKL). Osteoclast differentiation was evaluated via tartrate-resistant acid phosphatase (TRAP) staining and activity and F-actin ring formation. RANKL expression on synovial exosomes was assessed using flow cytometry and an enzyme-linked immunosorbent assay (ELISA). Synovial exosomes were the lowest in OA patients; these induced osteoclastogenesis in the absence of M-CSF and RANKL. Osteoclastogenesis was significantly higher with more exosomes in RA (p = 0.030) than in OA patients, but not in AS or gout patients. On treating macrophages with a specified number of synovial exosomes from RA/AS patients, exosomes induced greater osteoclastogenesis in RA than in AS patients. Synovial exosomal RANKL levels were significantly higher in RA (p = 0.035) than in AS patients. Synovial exosome numbers vary with the type of inflammatory arthritis. Synovial exosomes from RA patients may bear the disease-specific "synovial signature of osteoclastogenesis."
Keywords: inflammatory arthritis; osteoclastogenesis; synovial exosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Radner H., Ramiro S., Buchbinder R., Landewe R.B., van der Heijde D., Aletaha D. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst. Rev. 2012;1:CD008951. doi: 10.1002/14651858.CD008951.pub2. - DOI - PMC - PubMed
-
- Miyasaka N., Sato K., Goto M., Sasano M., Natsuyama M., Inoue K., Nishioka K. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Possible involvement in joint destruction. Arthritis Rheum. 1988;31:480–486. doi: 10.1002/art.1780310404. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

