K2 Transfection System Boosts the Adenoviral Transduction of Murine Mesenchymal Stromal Cells
- PMID: 33435318
- PMCID: PMC7826527
- DOI: 10.3390/ijms22020598
K2 Transfection System Boosts the Adenoviral Transduction of Murine Mesenchymal Stromal Cells
Abstract
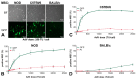
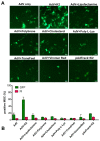
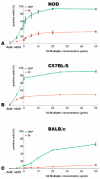
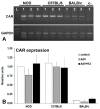
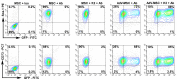
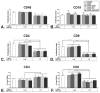
Adenoviral vectors are important vehicles for delivering therapeutic genes into mammalian cells. However, the yield of the adenoviral transduction of murine mesenchymal stromal cells (MSC) is low. Here, we aimed to improve the adenoviral transduction efficiency of bone marrow-derived MSC. Our data showed that among all the potential transduction boosters that we tested, the K2 Transfection System (K2TS) greatly increased the transduction efficiency. After optimization of both K2TS components, the yield of the adenoviral transduction increased from 18% to 96% for non-obese diabetic (NOD)-derived MSC, from 30% to 86% for C57BL/6-derived MSC, and from 0.6% to 63% for BALB/c-derived MSC, when 250 transduction units/cell were used. We found that MSC derived from these mouse strains expressed different levels of the coxsackievirus and adenovirus receptors (MSC from C57BL/6≥NOD>>>BALB/c). K2TS did not increase the level of the receptor expression, but desensitized the cells to foreign DNA and facilitated the virus entry into the cell. The expression of Stem cells antigen-1 (Sca-1) and 5'-nucleotidase (CD73) MSC markers, the adipogenic and osteogenic differentiation potential, and the immunosuppressive capacity were preserved after the adenoviral transduction of MSC in the presence of the K2TS. In conclusion, K2TS significantly enhanced the adenoviral transduction of MSC, without interfering with their main characteristics and properties.
Keywords: BALC/c-derived MSC; C57BL/6-derived MSC; K2 transfection system; NOD-derived MSC; adenovirus; mesenchymal stromal cells; transduction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Efficient adenoviral-mediated gene delivery into porcine mesenchymal stem cells.Mol Reprod Dev. 2006 Nov;73(11):1393-403. doi: 10.1002/mrd.20593. Mol Reprod Dev. 2006. PMID: 16897738
-
Polyethyleneimine-coating enhances adenoviral transduction of mesenchymal stem cells.Biochem Biophys Res Commun. 2014 May 9;447(3):383-7. doi: 10.1016/j.bbrc.2014.03.142. Epub 2014 Apr 12. Biochem Biophys Res Commun. 2014. PMID: 24727452
-
Adenoviral transduction of mesenchymal stem cells.Methods Mol Biol. 2007;407:265-74. doi: 10.1007/978-1-59745-536-7_18. Methods Mol Biol. 2007. PMID: 18453261
-
Comparison between adenoviral and retroviral vectors for the transduction of the thymidine kinase PET reporter gene in rat mesenchymal stem cells.J Nucl Med. 2008 Nov;49(11):1836-44. doi: 10.2967/jnumed.108.052175. J Nucl Med. 2008. PMID: 18984872
-
Efficient adenovirus-mediated gene transfer into primary T cells and thymocytes in a new coxsackie/adenovirus receptor transgenic model.BMC Immunol. 2002 May 2;3:4. doi: 10.1186/1471-2172-3-4. BMC Immunol. 2002. PMID: 12019030 Free PMC article.
Cited by
-
Transduction Enhancers Enable Efficient Human Adenovirus Type 5-Mediated Gene Transfer into Human Multipotent Mesenchymal Stromal Cells.Viruses. 2021 Jun 12;13(6):1136. doi: 10.3390/v13061136. Viruses. 2021. PMID: 34204818 Free PMC article.
-
Treatment with Mesenchymal Stromal Cells Overexpressing Fas-Ligand Ameliorates Acute Graft-versus-Host Disease in Mice.Int J Mol Sci. 2022 Jan 4;23(1):534. doi: 10.3390/ijms23010534. Int J Mol Sci. 2022. PMID: 35008964 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

