Animal Model Dependent Response to Pentagalloyl Glucose in Murine Abdominal Aortic Injury
- PMID: 33435461
- PMCID: PMC7827576
- DOI: 10.3390/jcm10020219
Animal Model Dependent Response to Pentagalloyl Glucose in Murine Abdominal Aortic Injury
Abstract
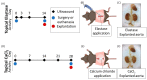
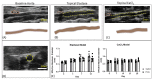
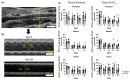
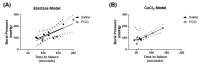
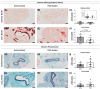
Abdominal aortic aneurysms (AAAs) are a local dilation of the aorta and are associated with significant mortality due to rupture and treatment complications. There is a need for less invasive treatments to prevent aneurysm growth and rupture. In this study, we used two experimental murine models to evaluate the potential of pentagalloyl glucose (PGG), which is a polyphenolic tannin that binds to and crosslinks elastin and collagen, to preserve aortic compliance. Animals underwent surgical aortic injury and received 0.3% PGG or saline treatment on the adventitial surface of the infrarenal aorta. Seventeen mice underwent topical elastase injury, and 14 mice underwent topical calcium chloride injury. We collected high-frequency ultrasound images before surgery and at 3-4 timepoints after. There was no difference in the in vivo effective maximum diameter due to PGG treatment for either model. However, the CaCl2 model had significantly higher Green-Lagrange circumferential cyclic strain in PGG-treated animals (p < 0.05). While ex vivo pressure-inflation testing showed no difference between groups in either model, histology revealed reduced calcium deposits in the PGG treatment group with the CaCl2 model. These findings highlight the continued need for improved understanding of PGG's effects on the extracellular matrix and suggest that PGG may reduce arterial calcium accumulation.
Keywords: abdominal aortic aneurysms; elastin; pentagalloyl glucose; ultrasound.
Conflict of interest statement
C.J.G. is a paid consultant of FUJIFILM VisualSonics. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Underlying Cause of Death, 1999–2015 Results Form. [(accessed on 27 August 2017)]; Available online: https://wonder.cdc.gov/controller/datarequest/D76;jsessionid=ED3A4ABA7F9....
-
- Benjamin E.J.M., Blaha M.J.M., Chiuve S.E.S., Cushman M.M., Das S.R.M., Deo R.M., de Ferranti S.D.M., Floyd J.M., Fornage M., Gillespie C.M., et al. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation. 2017;135 doi: 10.1161/CIR.0000000000000485. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

