Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance
- PMID: 33435513
- PMCID: PMC7827500
- DOI: 10.3390/ijms22020623
Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance
Abstract
Glucocorticoids (GCs) are steroids secreted by the adrenal cortex under the hypothalamic-pituitary-adrenal axis control, one of the major neuro-endocrine systems of the organism. These hormones are involved in tissue repair, immune stability, and metabolic processes, such as the regulation of carbohydrate, lipid, and protein metabolism. Globally, GCs are presented as 'flight and fight' hormones and, in that purpose, they are catabolic hormones required to mobilize storage to provide energy for the organism. If acute GC secretion allows fast metabolic adaptations to respond to danger, stress, or metabolic imbalance, long-term GC exposure arising from treatment or Cushing's syndrome, progressively leads to insulin resistance and, in fine, cardiometabolic disorders. In this review, we briefly summarize the pharmacological actions of GC and metabolic dysregulations observed in patients exposed to an excess of GCs. Next, we describe in detail the molecular mechanisms underlying GC-induced insulin resistance in adipose tissue, liver, muscle, and to a lesser extent in gut, bone, and brain, mainly identified by numerous studies performed in animal models. Finally, we present the paradoxical effects of GCs on beta cell mass and insulin secretion by the pancreas with a specific focus on the direct and indirect (through insulin-sensitive organs) effects of GCs. Overall, a better knowledge of the specific action of GCs on several organs and their molecular targets may help foster the understanding of GCs' side effects and design new drugs that possess therapeutic benefits without metabolic adverse effects.
Keywords: adipose tissue; glucocorticoids; insulin resistance; liver; muscle; pancreatic beta cells; signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
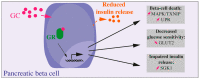
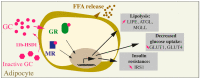
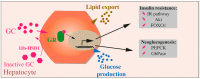


References
-
- Stewart P.M., Krozowski Z.S. 11 beta-Hydroxysteroid dehydrogenase. Vitam. Horm. 1999;57:249–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

