Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice
- PMID: 33435520
- PMCID: PMC7826900
- DOI: 10.3390/v13010086
Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice
Abstract
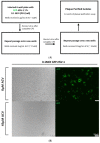
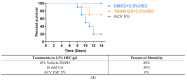
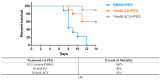
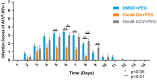
Herpes simplex virus type 1 (HSV-1) causes a lifelong latent infection with an estimated global prevalence of 66%. Primary and recurrent HSV infections are characterized by a tingling sensation, followed by an eruption of vesicles, which can cause painful erosions. Commonly used antiviral drugs against HSV infection are nucleoside analogues including acyclovir (ACV), famciclovir, and valacyclovir. Although these nucleoside analogues reduce morbidity and mortality in immunocompetent individuals, ACV-resistant HSV strains (ACVR-HSV) have been isolated from immunocompromised patients. Thus, ACVR-HSV infection poses a critical emerging public health concern. Recently, we reported that ginkgolic acid (GA) inhibits HSV-1 by disrupting viral structure, blocking fusion, and inhibiting viral protein synthesis. Additionally, we showed GA affords a broad spectrum of fusion inhibition of all three classes of fusion proteins, including those of HIV, Ebola, influenza A and Epstein Barr viruses. Here we report GA's antiviral activity against HSV-1 skin infection in BALB/cJ mice. GA-treated mice demonstrated a significantly reduced mortality rate and decreased infection scores compared to controls treated with dimethylsulfoxide (DMSO)-vehicle. Furthermore, GA efficiently inhibited ACVR-HSV-1 strain 17+ in vitro and in vivo. Since GA's mechanism of action includes virucidal activity and fusion inhibition, it is expected to work alone or synergistically with other anti-viral drugs, and we anticipate it to be effective against additional cutaneous and potentially systemic viral infections.
Keywords: acyclovir-resistance; antiviral; fusion inhibition; ginkgolic acid; herpes simplex type 1; virucidal activity; zosteriform infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Peptide Inhibitor of Complement C1, RLS-0071, Reduces Zosteriform Spread of Herpes Simplex Virus Type 1 Skin Infection and Promotes Survival in Infected Mice.Viruses. 2021 Jul 22;13(8):1422. doi: 10.3390/v13081422. Viruses. 2021. PMID: 34452288 Free PMC article.
-
Helicase primase inhibitors (HPIs) are efficacious for therapy of human herpes simplex virus (HSV) disease in an infection mouse model.Antiviral Res. 2021 Nov;195:105190. doi: 10.1016/j.antiviral.2021.105190. Epub 2021 Oct 16. Antiviral Res. 2021. PMID: 34666109
-
Ginkgolic acid inhibits fusion of enveloped viruses.Sci Rep. 2020 Mar 16;10(1):4746. doi: 10.1038/s41598-020-61700-0. Sci Rep. 2020. PMID: 32179788 Free PMC article.
-
Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management.Antimicrob Agents Chemother. 2011 Feb;55(2):459-72. doi: 10.1128/AAC.00615-10. Epub 2010 Nov 15. Antimicrob Agents Chemother. 2011. PMID: 21078929 Free PMC article. Review.
-
[Anti alpha-herpesvirus drugs].Nihon Rinsho. 2012 Apr;70(4):558-63. Nihon Rinsho. 2012. PMID: 22568134 Review. Japanese.
Cited by
-
Hepatitis B and Hepatitis D Viruses: A Comprehensive Update with an Immunological Focus.Int J Mol Sci. 2022 Dec 15;23(24):15973. doi: 10.3390/ijms232415973. Int J Mol Sci. 2022. PMID: 36555623 Free PMC article. Review.
-
Sophoridine Suppresses Herpes Simplex Virus Type 1 Infection by Blocking the Activation of Cellular PI3K/Akt and p38 MAPK Pathways.Front Microbiol. 2022 Jun 10;13:872505. doi: 10.3389/fmicb.2022.872505. eCollection 2022. Front Microbiol. 2022. PMID: 35756044 Free PMC article.
-
Natural Products and Their Derivatives against Human Herpesvirus Infection.Molecules. 2021 Oct 18;26(20):6290. doi: 10.3390/molecules26206290. Molecules. 2021. PMID: 34684870 Free PMC article. Review.
-
Insights into Antiviral Properties and Molecular Mechanisms of Non-Flavonoid Polyphenols against Human Herpesviruses.Int J Mol Sci. 2022 Nov 11;23(22):13891. doi: 10.3390/ijms232213891. Int J Mol Sci. 2022. PMID: 36430369 Free PMC article. Review.
-
Ginkgo biloba in the management of the COVID-19 severity.Arch Pharm (Weinheim). 2022 Oct;355(10):e2200188. doi: 10.1002/ardp.202200188. Epub 2022 Jun 7. Arch Pharm (Weinheim). 2022. PMID: 35672257 Free PMC article. Review.
References
-
- CDC Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14–49: United States, 2015–2016 (Number 304; February 2018) [(accessed on 11 July 2020)]; Available online: https://www.cdc.gov/nchs/products/databriefs/db304.htm#:~:text=The%20pre....
-
- Lundberg P., Ramakrishna C., Brown J., Tyszka J.M., Hamamura M., Hinton D.R., Kovats S., Nalcioglu O., Weinberg K., Openshaw H. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J. Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. - DOI - PMC - PubMed
-
- Piret J., Désormeaux A., Gourde P., Juhász J., Bergeron M.G. Efficacies of topical formulations of foscarnet and acyclovir and of 5-percent acyclovir ointment (Zovirax) in a murine model of cutaneous herpes simplex virus type 1 infection. Antimicrob. Agents Chemother. 2000;44:30–38. doi: 10.1128/AAC.44.1.30-38.2000. - DOI - PMC - PubMed
-
- Van Lint A., Ayers M., Brooks A.G., Coles R.M., Heath W.R., Carbone F.R. Herpes Simplex Virus-Specific CD8 T Cells Can Clear Established Lytic Infections from Skin and Nerves and Can Partially Limit the Early Spread of Virus after Cutaneous Inoculation. J. Immunol. 2004;172:392–397. doi: 10.4049/jimmunol.172.1.392. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

