Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis
- PMID: 33435542
- PMCID: PMC7827869
- DOI: 10.3390/ani11010133
Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis
Abstract
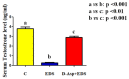
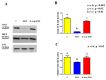
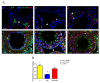
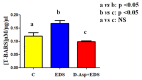
Herein is reported the first evidence of the protective role of D-aspartic acid (D-Asp) in preventing the toxic effect exerted by the alkylating agent ethane dimethane sulfonate (EDS) in the rat testis. We confirmed that EDS treatment specifically destroyed Leydig cells (LC), resulting in the drastic decrease of the serum testosterone level and producing morphological changes in the germinal tubules, i.e., altered organization of the epithelium, loss of cell contacts and the consequent presence of empty spaces between them, and a reduce number of spermatozoa. Moreover, an increase of TUNEL-positive germ cells, other than alteration in the protein level and localization of two LC "markers", StAR and PREP, were observed. Interestingly, results obtained from rats pre-treated with D-Asp for 15 days before EDS-injection showed that all the considered parameters were quite normal. To explore the probable mechanism(s) involved in the protection exerted by D-Asp, we considered the increased oxidative stress induced by EDS and the D-Asp antioxidant effects. Thiobarbiturc acid-reactive species (TBARS) levels increased following EDS-injection, while no change was observed in the D-Asp + EDS treated rats. Our results showed that D-Asp may be used as a strategy to mitigate the toxic effects exerted by environmental pollutants, as endocrine disrupters, in order to preserve the reproductive function.
Keywords: D-aspartic acid; EDS; PREP; StAR; endocrine disrupters; testis; testosterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Localization of D-aspartic acid in elongate spermatids in rat testis.Arch Biochem Biophys. 1998 Mar 1;351(1):96-105. doi: 10.1006/abbi.1997.0539. Arch Biochem Biophys. 1998. PMID: 9500846
-
The pattern of inhibin/activin alpha- and betaB-subunit messenger ribonucleic acid expression in rat testis after selective Leydig cell destruction by ethylene dimethane sulfonate.Endocrinology. 1999 Dec;140(12):5761-70. doi: 10.1210/endo.140.12.7193. Endocrinology. 1999. PMID: 10579342
-
Leukocyte populations of the adult rat testis following removal of the Leydig cells by treatment with ethane dimethane sulfonate and subcutaneous testosterone implants.Biol Reprod. 1994 Sep;51(3):551-61. doi: 10.1095/biolreprod51.3.551. Biol Reprod. 1994. PMID: 7528551
-
Spermatogenesis by Sisyphus: proliferating stem germ cells fail to repopulate the testis after 'irreversible' injury.Adv Exp Med Biol. 2001;500:421-8. doi: 10.1007/978-1-4615-0667-6_64. Adv Exp Med Biol. 2001. PMID: 11764975 Review.
-
Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement.Am J Anat. 1990 May;188(1):3-20. doi: 10.1002/aja.1001880103. Am J Anat. 1990. PMID: 2161173 Review.
Cited by
-
First Evidence of the Expression and Localization of Prothymosin α in Human Testis and Its Involvement in Testicular Cancers.Biomolecules. 2022 Aug 31;12(9):1210. doi: 10.3390/biom12091210. Biomolecules. 2022. PMID: 36139050 Free PMC article.
-
Oral D-Aspartate Treatment Improves Sperm Fertility in Both Young and Adult B6N Mice.Animals (Basel). 2022 May 25;12(11):1350. doi: 10.3390/ani12111350. Animals (Basel). 2022. PMID: 35681815 Free PMC article.
-
The simultaneous administration of microplastics and cadmium alters rat testicular activity and changes the expression of PTMA, DAAM1 and PREP.Front Cell Dev Biol. 2023 Mar 9;11:1145702. doi: 10.3389/fcell.2023.1145702. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36968197 Free PMC article.
-
A short-term high-fat diet alters rat testicular activity and blood-testis barrier integrity through the SIRT1/NRF2/MAPKs signaling pathways.Front Endocrinol (Lausanne). 2023 Oct 27;14:1274035. doi: 10.3389/fendo.2023.1274035. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38027181 Free PMC article.
-
Type 1 diabetes impairs the activity of rat testicular somatic and germ cells through NRF2/NLRP3 pathway-mediated oxidative stress.Front Endocrinol (Lausanne). 2024 May 16;15:1399256. doi: 10.3389/fendo.2024.1399256. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38818504 Free PMC article.
References
-
- Ergoli M., Venditti M., Piccillo E., Minucci S., Politano L. Study of Expression of Genes Potentially Responsible for Reduced Fitness in Patients with Myotonic Dystrophy Type 1 and Identification of New Biomarkers of Testicular Function. Mol. Reprod. Dev. 2020;87:45–52. doi: 10.1002/mrd.23307. - DOI - PubMed
-
- Grive K.J., Hu Y., Shu E., Grimson A., Elemento O., Grenier J.K., Cohen P.E. Dynamic transcriptome profiles within spermatogonial and spermatocyte populations during postnatal testis maturation revealed by single-cell sequencing. PLoS Genetics. 2019;15:1007810. doi: 10.1371/journal.pgen.1007810. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

