Human Keratinocytes Adopt Neuronal Fates After In Utero Transplantation in the Developing Rat Brain
- PMID: 33435710
- PMCID: PMC7809298
- DOI: 10.1177/0963689720978219
Human Keratinocytes Adopt Neuronal Fates After In Utero Transplantation in the Developing Rat Brain
Abstract
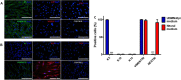
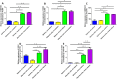
Human skin contains keratinocytes in the epidermis. Such cells share their ectodermal origin with the central nervous system (CNS). Recent studies have demonstrated that terminally differentiated somatic cells can adopt a pluripotent state, or can directly convert its phenotype to neurons, after ectopic expression of transcription factors. In this article we tested the hypothesis that human keratinocytes can adopt neural fates after culturing them in suspension with a neural medium. Initially, keratinocytes expressed Keratins and Vimentin. After neural induction, transcriptional upregulation of NESTIN, SOX2, VIMENTIN, SOX1, and MUSASHI1 was observed, concomitant with significant increases in NESTIN detected by immunostaining. However, in vitro differentiation did not yield the expression of neuronal or astrocytic markers. We tested the differentiation potential of control and neural-induced keratinocytes by grafting them in the developing CNS of rats, through ultrasound-guided injection. For this purpose, keratinocytes were transduced with lentivirus that contained the coding sequence of green fluorescent protein. Cell sorting was employed to select cells with high fluorescence. Unexpectedly, 4 days after grafting these cells in the ventricles, both control and neural-induced cells expressed green fluorescent protein together with the neuronal proteins βIII-Tubulin and Microtubule-Associated Protein 2. These results support the notion that in vivo environment provides appropriate signals to evaluate the neuronal differentiation potential of keratinocytes or other non-neural cell populations.
Keywords: FACS sorting; epidermis; human skin; ultrasound-guided grafting.
Conflict of interest statement
Figures





Similar articles
-
Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone.J Neurosci Res. 2000 Feb 1;59(3):321-31. doi: 10.1002/(sici)1097-4547(20000201)59:3<321::aid-jnr5>3.0.co;2-9. J Neurosci Res. 2000. PMID: 10679767
-
De-differentiation of mouse interfollicular keratinocytes by the embryonic transcription factor Oct-4.J Invest Dermatol. 2007 Feb;127(2):372-80. doi: 10.1038/sj.jid.5700531. Epub 2006 Aug 24. J Invest Dermatol. 2007. PMID: 16932739
-
Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study.Lancet. 2009 Nov 21;374(9703):1745-53. doi: 10.1016/S0140-6736(09)61496-3. Lancet. 2009. PMID: 19932355
-
[Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French.
-
Neural precursor cells: applications for the study and repair of the central nervous system.Neurobiol Dis. 1997;4(1):1-22. doi: 10.1006/nbdi.1997.0137. Neurobiol Dis. 1997. PMID: 9258907 Review.
References
-
- Martín-Ibáñez R, Guardia I, Pardo M, Herranz C, Zietlow R, Vinh N-N, Rosser A, Canals JM. Insights in spatio-temporal characterization of human fetal neural stem cells. Exp Neurol. 2017;291:20–35. - PubMed
-
- Schwartz PH, Bryant PJ, Fuja TJ, Su H, O’Dowd DK, Klassen H. Isolation and Characterization of Neural Progenitor Cells from Post-Mortem Human Cortex. J Neurosci Res. 2003;74(6):838–851. - PubMed
-
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. - PubMed
-
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. - PubMed
-
- Féron F, Perry C, Girard SD, Mackay-Sim A. Isolation of Adult Stem Cells from the Human Olfactory Mucosa In: Reynolds BA, Deleyrolle LP, eds. Neural Progenitor Cells: Methods and Protocols; Methods in Molecular Biology. Vol. 1059 New York (NY): Neural Pro. Springer Science+Business; 2013:107–114. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

