COVID-19 vaccine: where are we now and where should we go?
- PMID: 33435774
- PMCID: PMC7898300
- DOI: 10.1080/14760584.2021.1875824
COVID-19 vaccine: where are we now and where should we go?
Abstract
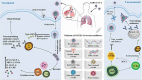
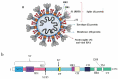
Introduction: The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has currently caused the pandemic with a high progressive speed and has been considered as the global public health crisis in 2020. This new member of the coronavirus family has created a potentially fatal disease, called coronavirus disease-2019 (COVID-19). Despite the continuous efforts of researchers to find effective vaccines and drugs for COVID-19, there is still no success in this matter.
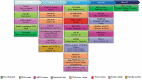
Areas covered: Here, the literature regarding the COVID-19 vaccine candidates currently in the clinical trials, as well as main candidates in pre-clinical stages for development and research, were reviewed. These candidates have been developed under five different major platforms, including live-attenuated vaccine, mRNA-based vaccine, DNA vaccines, inactivated virus, and viral-vector-based vaccine.
Expert opinion: There are several limitations in the field of the rapid vaccine development against SARS-CoV-2, and other members of the coronavirus family such as SARS-CoV and MERS-CoV. The key challenges of designing an effective vaccine within a short time include finding the virulence ability of an emerging virus and potential antigen, choosing suitable experimental models and efficient route of administration, the immune-response study, designing the clinical trials, and determining the safety, as well as efficacy.
Keywords: SARS-CoV-2; clinical trials; covid-19; vaccine.
Figures
References
-
- Cyranoski D. Did pangolins spread the China coronavirus to people [Internet]. editor. Heidelberg: Springer Nature; 2020;502. - PubMed
-
- Hopkins J Corona virus resource center. Im Internet (Stand: 19.04. 2020): https://coronavirus. jhu. edu/data, (2020).
-
- Organization WH .DRAFT landscape of COVID-19 candidate vaccines.World Health Organization.https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-lan..., (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
