Gut microbiota: impacts on gastrointestinal cancer immunotherapy
- PMID: 33435800
- PMCID: PMC7808428
- DOI: 10.1080/19490976.2020.1869504
Gut microbiota: impacts on gastrointestinal cancer immunotherapy
Abstract
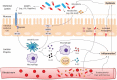
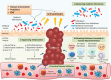
The association of gut microbiota with gastrointestinal carcinogenesis has been heavily investigated since the recent advance in sequencing technology. Accumulating evidence has revealed the critical roles of commensal microbes in cancer progression. Given by its importance, emerging studies have focussed on targeting microbiota to ameliorate therapeutic effectiveness. It is now clear that the microbial community is closely related to the efficacy of chemotherapy, while the correlation of microbiota with immunotherapy is much less studied. Herein, we review the up-to-date findings on the influence of gut microbiota on three common immunotherapies including adoptive cell transfer, immune checkpoint blockade, and CpG-oligodeoxynucleotide therapy. We then explore three microbiota-targeted strategies that may improve treatment efficacy, involving dietary intervention, probiotics supplementation, and fecal microbiota transplantation.
Keywords: CpG-oligodeoxynucleotide therapy; Gut microbiota; adoptive cell transfer; blockade-induced adverse events; fecal microbiota transplantation; gastrointestinal cancer; immune checkpoint blockade; probiotics.
Figures
Similar articles
-
The role of the gut microbiota in tumor, immunity, and immunotherapy.Front Immunol. 2024 Jun 5;15:1410928. doi: 10.3389/fimmu.2024.1410928. eCollection 2024. Front Immunol. 2024. PMID: 38903520 Free PMC article. Review.
-
Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus spp. and Bifidobacterium spp. in Gastrointestinal Cancers.Nutrients. 2021 Jul 31;13(8):2674. doi: 10.3390/nu13082674. Nutrients. 2021. PMID: 34444834 Free PMC article. Review.
-
Modulation of Gut Microbiota to Enhance Effect of Checkpoint Inhibitor Immunotherapy.Front Immunol. 2021 Jun 29;12:669150. doi: 10.3389/fimmu.2021.669150. eCollection 2021. Front Immunol. 2021. PMID: 34267748 Free PMC article. Review.
-
Gut microbiota as a biomarker and modulator of anti-tumor immunotherapy outcomes.Front Immunol. 2024 Nov 28;15:1471273. doi: 10.3389/fimmu.2024.1471273. eCollection 2024. Front Immunol. 2024. PMID: 39669573 Free PMC article. Review.
-
Gut Microbiome Modulation Via Fecal Microbiota Transplant to Augment Immunotherapy in Patients with Melanoma or Other Cancers.Curr Oncol Rep. 2020 Jun 24;22(7):74. doi: 10.1007/s11912-020-00913-y. Curr Oncol Rep. 2020. PMID: 32577835 Free PMC article. Review.
Cited by
-
The Influence of the Microbiome on Immunotherapy for Gastroesophageal Cancer.Cancers (Basel). 2023 Sep 5;15(18):4426. doi: 10.3390/cancers15184426. Cancers (Basel). 2023. PMID: 37760397 Free PMC article. Review.
-
Strategies for treating the cold tumors of cholangiocarcinoma: core concepts and future directions.Clin Exp Med. 2024 Aug 14;24(1):193. doi: 10.1007/s10238-024-01460-7. Clin Exp Med. 2024. PMID: 39141161 Free PMC article. Review.
-
Local Delivery of Immunomodulatory Antibodies for Gastrointestinal Tumors.Cancers (Basel). 2023 Apr 18;15(8):2352. doi: 10.3390/cancers15082352. Cancers (Basel). 2023. PMID: 37190279 Free PMC article. Review.
-
Diet-gut microbial interactions influence cancer immunotherapy.Front Oncol. 2023 Mar 24;13:1138362. doi: 10.3389/fonc.2023.1138362. eCollection 2023. Front Oncol. 2023. PMID: 37035188 Free PMC article. Review.
-
Dietary oxidized beef protein alters gut microbiota and induces colonic inflammatory damage in C57BL/6 mice.Front Nutr. 2022 Sep 2;9:980204. doi: 10.3389/fnut.2022.980204. eCollection 2022. Front Nutr. 2022. PMID: 36118776 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
