Adjuvant albumin-bound paclitaxel combined with S-1 vs. oxaliplatin combined with capecitabine after D2 gastrectomy in patients with stage III gastric adenocarcinoma: a phase III multicenter, open-label, randomized controlled clinical trial protocol
- PMID: 33435909
- PMCID: PMC7802165
- DOI: 10.1186/s12885-020-07772-7
Adjuvant albumin-bound paclitaxel combined with S-1 vs. oxaliplatin combined with capecitabine after D2 gastrectomy in patients with stage III gastric adenocarcinoma: a phase III multicenter, open-label, randomized controlled clinical trial protocol
Abstract
Background: Surgery is the only treatment option for operable gastric cancer. The CLASSIC and ACTS-GC studies showed that the 5-year overall survival (OS) of patients with stage III gastric cancer undergoing D2 gastrectomy is still very low. Whether adjuvant nanoparticle albumin-bound paclitaxel (nab-paclitaxel) combined chemotherapy is more effective than the XELOX standard adjuvant chemotherapy in patients with stage III gastric cancer has not been confirmed.
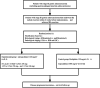
Methods: This is a multicenter, open-label, phase III clinical study. In this trial, 616 patients with locally advanced stage III gastric cancer that underwent curative D2 radical surgery and achieved R0 are planned to be included. Patients will be randomized 1:1 to nab-paclitaxel combined with S-1 (AS) vs. oxaliplatin combined with capecitabine (XELOX). XELOX group: Patients assigned to the XELOX group received eight 3-week cycles of oral capecitabine (1000 mg/m2) twice daily on days 1-14 of each cycle plus intravenous oxaliplatin 130 mg/m2 on day 1 of each cycle. AS group: AS group received eight 3-week cycles of oral S-1 (80-120 mg) (< 1.25 m2, 40 mg; 1.25 to < 1.5 m2, 50 mg; and > 1.5 m2, 60 mg) twice daily on days 1-14 plus intravenous nab-paclitaxel 120 mg/m2 on days 1 and 8 of each cycle. The primary endpoint was the 3-year disease-free survival (3-year-DFS) defined as the time from randomisation to the time of recurrence of the original gastric cancer, development of a new gastric cancer, or death from any cause. The secondary endpoints were the overall survival, (defined as the time from the date of randomisation to date of death from any cause) and safety (any adverse event).
Discussion: Compared with previous studies, this study includes nab-paclitaxel based on S-1 adjuvant chemotherapy, which is expected to achieve better efficacy and lower toxicity than the standard treatment. This study is the first clinical study to evaluate the safety and efficacy of nab-paclitaxel combined with S-1 in patients with stage III gastric cancer after D2 radical resection.
Trial registration: This clinical trial has been registered with ClinicalTrials.gov, registration number: NCT04135781 , on October 20th, 2019.
Keywords: Adjuvant chemotherapy; Albumin-bound paclitaxel; Gastric cancer; S-1; Surgery; Survival.
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Zuo TZR, Zeng H, Zhang S, Chen W. Epidemiology of stomach cancer in China. Chin J Clin Oncol. 2017;44:52–58.
-
- Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. https://www.ncbi.nlm.nih.gov/books/NBK487794/. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous