Targeting hypoxic tumor microenvironment in pancreatic cancer
- PMID: 33436044
- PMCID: PMC7805044
- DOI: 10.1186/s13045-020-01030-w
Targeting hypoxic tumor microenvironment in pancreatic cancer
Abstract
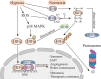
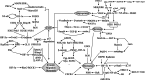
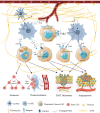
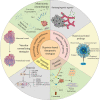
Attributable to its late diagnosis, early metastasis, and poor prognosis, pancreatic cancer remains one of the most lethal diseases worldwide. Unlike other solid tumors, pancreatic cancer harbors ample stromal cells and abundant extracellular matrix but lacks vascularization, resulting in persistent and severe hypoxia within the tumor. Hypoxic microenvironment has extensive effects on biological behaviors or malignant phenotypes of pancreatic cancer, including metabolic reprogramming, cancer stemness, invasion and metastasis, and pathological angiogenesis, which synergistically contribute to development and therapeutic resistance of pancreatic cancer. Through various mechanisms including but not confined to maintenance of redox homeostasis, activation of autophagy, epigenetic regulation, and those induced by hypoxia-inducible factors, intratumoral hypoxia drives the above biological processes in pancreatic cancer. Recognizing the pivotal roles of hypoxia in pancreatic cancer progression and therapies, hypoxia-based antitumoral strategies have been continuously developed over the recent years, some of which have been applied in clinical trials to evaluate their efficacy and safety in combinatory therapies for patients with pancreatic cancer. In this review, we discuss the molecular mechanisms underlying hypoxia-induced aggressive and therapeutically resistant phenotypes in both pancreatic cancerous and stromal cells. Additionally, we focus more on innovative therapies targeting the tumor hypoxic microenvironment itself, which hold great potential to overcome the resistance to chemotherapy and radiotherapy and to enhance antitumor efficacy and reduce toxicity to normal tissues.
Keywords: Angiogenesis; Autophagy; EMT and metastasis; Hypoxia; Innovative therapies; Metabolic reprogramming; Pancreatic cancer; Redox homeostasis; Stemness; Therapeutic resistance.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Leonhardt CS, Traub B, Hackert T, Klaiber U, Strobel O, Büchler MW, et al. Adjuvant and neoadjuvant chemotherapy in pancreatic ductal adenocarcinoma. J Pancreatol. 2020;3:1–11. doi: 10.1097/JP9.0000000000000040. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

