Immune Skew of Circulating Follicular Helper T Cells Associates With Myasthenia Gravis Severity
- PMID: 33436376
- PMCID: PMC8105905
- DOI: 10.1212/NXI.0000000000000945
Immune Skew of Circulating Follicular Helper T Cells Associates With Myasthenia Gravis Severity
Abstract
Objective: To clarify functional alterations of follicular helper T cells (Tfh) in myasthenia gravis (MG) because Tfh play important roles in helping B cells generate antibody-producing cells.
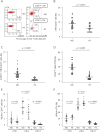
Methods: A total of 24 immunotherapy-naive patients with anti-acetylcholine receptor (AchR) antibody-positive MG and 18 age-matched healthy subjects (HS) were enrolled. Samples from 6 patients were available for posttreatment analysis. Subsets of circulating Tfh (cTfh) and B cells were identified by flow cytometry analysis of surface molecules. Cytokine production by isolated cTfh subsets from 5 patients with MG and 5 HS was measured in vitro. Analysis was performed to examine the correlation between the frequency of cTfh subsets and that of plasmablasts and between cTfh subsets and the quantitative MG score.
Results: cTfh increased with elevated expression of inducible T-cell costimulator (ICOS) in patients with MG. cTfh shifted to Th2 and Th17 over Th1 in MG. ICOShighcTfh produced significantly higher levels of interleukin (IL)-21, IL-4, and IL-17A than ICOSlow cTfh only in patients with MG. The frequency of cTfh within CD4 T cells was more closely associated with disease severity than the serum anti-AchR antibody titer and frequency of plasmablasts within B cells. Abnormalities of cTfh were improved after immunotherapy in parallel with clinical improvement.
Conclusions: Alternation of cTfh is a key feature in the development of MG and may become a biomarker for disease severity and therapeutic efficacy.
Classification of evidence: This study provides Class II evidence that the level of cTfh is associated with disease severity in patients with MG.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


References
-
- Gilhus N, Verschuuren J. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015;14:1023–1036. - PubMed
-
- Aurangzeb S, Tariq M, Irshad M, Badshah M, Khan RS. Relationship between anti-acetylcholine receptor antibody titres and severity of myasthenia gravis. J Pak Med Assoc 2009;59:289–292. - PubMed
-
- Lindstrom J, Seybold M, Lennon V, Whittingham S, Duane D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 1976;26:1054–1059. - PubMed
-
- Oosterhuis HJ, Limburg PC, Hummel-Tappel E, The TH. Anti-acetylcholine receptor antibodies in myasthenia gravis. Part 2. Clinical and serological follow-up of individual patients. J Neurol Sci 1983;58:371–385. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
