Ferric heme as a CO/NO sensor in the nuclear receptor Rev-Erbß by coupling gas binding to electron transfer
- PMID: 33436410
- PMCID: PMC7826342
- DOI: 10.1073/pnas.2016717118
Ferric heme as a CO/NO sensor in the nuclear receptor Rev-Erbß by coupling gas binding to electron transfer
Abstract
Rev-Erbβ is a nuclear receptor that couples circadian rhythm, metabolism, and inflammation. Heme binding to the protein modulates its function as a repressor, its stability, its ability to bind other proteins, and its activity in gas sensing. Rev-Erbβ binds Fe3+-heme more tightly than Fe2+-heme, suggesting its activities may be regulated by the heme redox state. Yet, this critical role of heme redox chemistry in defining the protein's resting state and function is unknown. We demonstrate by electrochemical and whole-cell electron paramagnetic resonance experiments that Rev-Erbβ exists in the Fe3+ form within the cell allowing the protein to be heme replete even at low concentrations of labile heme in the nucleus. However, being in the Fe3+ redox state contradicts Rev-Erb's known function as a gas sensor, which dogma asserts must be Fe2+ This paper explains why the resting Fe3+ state is congruent both with heme binding and cellular gas sensing. We show that the binding of CO/NO elicits a striking increase in the redox potential of the Fe3+/Fe2+ couple, characteristic of an EC mechanism in which the unfavorable Electrochemical reduction of heme is coupled to the highly favorable Chemical reaction of gas binding, making the reduction spontaneous. Thus, Fe3+-Rev-Erbβ remains heme-loaded, crucial for its repressor activity, and undergoes reduction when diatomic gases are present. This work has broad implications for proteins in which ligand-triggered redox changes cause conformational changes influencing its function or interprotein interactions (e.g., between NCoR1 and Rev-Erbβ). This study opens up the possibility of CO/NO-mediated regulation of the circadian rhythm through redox changes in Rev-Erbβ.
Keywords: Rev-Erb; electro-chemical coupling; heme; redox.
Conflict of interest statement
The authors declare no competing interest.
Figures
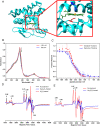
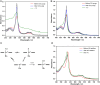

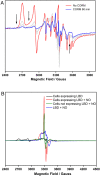
References
-
- Kumar Jha P., Challet E., Kalsbeek A., Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell. Endocrinol. 418, 74–88 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

