SIRT6 promotes angiogenesis and hemorrhage of carotid plaque via regulating HIF-1α and reactive oxygen species
- PMID: 33436551
- PMCID: PMC7804142
- DOI: 10.1038/s41419-020-03372-2
SIRT6 promotes angiogenesis and hemorrhage of carotid plaque via regulating HIF-1α and reactive oxygen species
Abstract
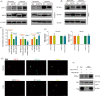
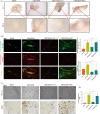
As a member of Sirtuins family, SIRT6 participates in the physiological and pathological progress of DNA repair, anti-aging, metabolism, and so on. Several studies have demonstrated that knockdown of SIRT6 inhibited the development of atherosclerosis (AS), indicated SIRT6 as a protective factor for AS. However, we confirmed SIRT6 was significantly overexpressed in human unstable carotid plaques compared with stable carotid plaques. This result indicated a more complex role of SIRT6 in AS. Furthermore, we constructed mice model with unstable carotid plaque and injected them with SIRT6 overexpressed adeno-associated virus (AAV-SIRT6). AAV-SIRT6 significantly promoted angiogenesis as well as hemorrhage in plaques. In vitro, we demonstrated overexpression of SIRT6 prevented HIF-1α from degradation by deubiquitination at K37 and K532 of HIF-1α, thus promoted the expression of HIF-1α under both normoxia and hypoxia in human umbilical vein endothelial cells (HUVECs). Through regulating HIF-1α, overexpression of SIRT6 promoted invasion, migration, proliferation, as well as tube formation ability of HUVECs. Interestingly, under different conditions, SIRT6 played different roles in the function of HUVECs. Under oxidative stress, another important pathological environment for AS, SIRT6 bound to the promoter of Catalase, a main reactive oxygen species scavenger, and depleted H3K56 acetylation, thus inhibited expression and activity of Catalase at the transcriptional level. Subsequently, inhibited Catalase promoted reactive oxygen species (ROS) under oxidative stress. Accumulated ROS further aggravated oxidative stress injury of HUVECs. On one hand, SIRT6 promoted angiogenesis in plaque via HIF-1α under hypoxia. On the other hand, SIRT6 promoted injury of neovascular via ROS under oxidative stress. It is this process of continuous growth and damage that leads to hemorrhage in carotid plaque. In conclusion, we innovatively confirmed SIRT6 promoted the angiogenesis and IPH via promoting HIF-1α and ROS in different environments, thus disclosed the unknowing danger of SIRT6.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Os, H. J. A. V., Mulder, I. A., Broersen, A., Algra, A. & Wermer, M. J. H. Migraine and cerebrovascular atherosclerosis in patients with ischemic stroke. Stroke48, 1973–1975 (2017). - PubMed
-
- Loftus, I. M. Intraplaque Hemorrhage and Progression of Coronary Atheroma. N. Engl. J. Med. 349, 2316–2325 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

