Structure, function, and pathology of protein O-glucosyltransferases
- PMID: 33436558
- PMCID: PMC7803782
- DOI: 10.1038/s41419-020-03314-y
Structure, function, and pathology of protein O-glucosyltransferases
Abstract
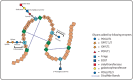
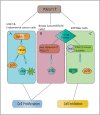
Protein O-glucosylation is a crucial form of O-glycosylation, which involves glucose (Glc) addition to a serine residue within a consensus sequence of epidermal growth factor epidermal growth factor (EGF)-like repeats found in several proteins, including Notch. Glc provides stability to EGF-like repeats, is required for S2 cleavage of Notch, and serves to regulate the trafficking of Notch, crumbs2, and Eyes shut proteins to the cell surface. Genetic and biochemical studies have shown a link between aberrant protein O-glucosylation and human diseases. The main players of protein O-glucosylation, protein O-glucosyltransferases (POGLUTs), use uridine diphosphate (UDP)-Glc as a substrate to modify EGF repeats and reside in the endoplasmic reticulum via C-terminal KDEL-like signals. In addition to O-glucosylation activity, POGLUTs can also perform protein O-xylosylation function, i.e., adding xylose (Xyl) from UDP-Xyl; however, both activities rely on residues of EGF repeats, active-site conformations of POGLUTs and sugar substrate concentrations in the ER. Impaired expression of POGLUTs has been associated with initiation and progression of human diseases such as limb-girdle muscular dystrophy, Dowling-Degos disease 4, acute myeloid leukemia, and hepatocytes and pancreatic dysfunction. POGLUTs have been found to alter the expression of cyclin-dependent kinase inhibitors (CDKIs), by affecting Notch or transforming growth factor-β1 signaling, and cause cell proliferation inhibition or induction depending on the particular cell types, which characterizes POGLUT's cell-dependent dual role. Except for a few downstream elements, the precise mechanisms whereby aberrant protein O-glucosylation causes diseases are largely unknown, leaving behind many questions that need to be addressed. This systemic review comprehensively covers literature to understand the O-glucosyltransferases with a focus on POGLUT1 structure and function, and their role in health and diseases. Moreover, this study also raises unanswered issues for future research in cancer biology, cell communications, muscular diseases, etc.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures




References
-
- Zachara, N., Akimoto, Y. & Hart, G. W. in Essentials of Glycobiology. 3rd edn (Cold Sping Harbor, NY, 2017).
-
- Hart, G. W. & Akimoto, Y. in Essentials of Glycobiology. 2nd edn (Cold Sping Harbor, NY, 2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

