The power of electrified nanoconfinement for energising, controlling and observing long enzyme cascades
- PMID: 33436601
- PMCID: PMC7804111
- DOI: 10.1038/s41467-020-20403-w
The power of electrified nanoconfinement for energising, controlling and observing long enzyme cascades
Abstract
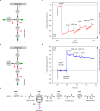
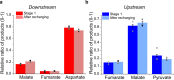
Multistep enzyme-catalyzed cascade reactions are highly efficient in nature due to the confinement and concentration of the enzymes within nanocompartments. In this way, rates are exceptionally high, and loss of intermediates minimised. Similarly, extended enzyme cascades trapped and crowded within the nanoconfined environment of a porous conducting metal oxide electrode material form the basis of a powerful way to study and exploit myriad complex biocatalytic reactions and pathways. One of the confined enzymes, ferredoxin-NADP+ reductase, serves as a transducer, rapidly and reversibly recycling nicotinamide cofactors electrochemically for immediate delivery to the next enzyme along the chain, thereby making it possible to energize, control and observe extended cascade reactions driven in either direction depending on the electrode potential that is applied. Here we show as proof of concept the synthesis of aspartic acid from pyruvic acid or its reverse oxidative decarboxylation/deamination, involving five nanoconfined enzymes.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
NADP(H)-dependent biocatalysis without adding NADP(H).Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2214123120. doi: 10.1073/pnas.2214123120. Epub 2022 Dec 27. Proc Natl Acad Sci U S A. 2023. PMID: 36574703 Free PMC article.
-
From Protein Film Electrochemistry to Nanoconfined Enzyme Cascades and the Electrochemical Leaf.Chem Rev. 2023 May 10;123(9):5421-5458. doi: 10.1021/acs.chemrev.2c00397. Epub 2022 Dec 27. Chem Rev. 2023. PMID: 36573907 Free PMC article. Review.
-
Progress in Scaling up and Streamlining a Nanoconfined, Enzyme-Catalyzed Electrochemical Nicotinamide Recycling System for Biocatalytic Synthesis.ChemElectroChem. 2020 Nov 16;7(22):4672-4678. doi: 10.1002/celc.202001166. Epub 2020 Nov 20. ChemElectroChem. 2020. PMID: 33381377 Free PMC article.
-
Building Localized NADP(H) Recycling Circuits to Advance Enzyme Cascadetronics.Angew Chem Int Ed Engl. 2025 Mar 3;64(10):e202414176. doi: 10.1002/anie.202414176. Epub 2025 Feb 11. Angew Chem Int Ed Engl. 2025. PMID: 39876743 Free PMC article.
-
Magneto-controlled enzyme reactions.Methods Enzymol. 2020;630:1-24. doi: 10.1016/bs.mie.2019.09.002. Epub 2019 Oct 31. Methods Enzymol. 2020. PMID: 31931981 Review.
Cited by
-
NADP(H)-dependent biocatalysis without adding NADP(H).Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2214123120. doi: 10.1073/pnas.2214123120. Epub 2022 Dec 27. Proc Natl Acad Sci U S A. 2023. PMID: 36574703 Free PMC article.
-
Osmotic energy conversion in serpentinite-hosted deep-sea hydrothermal vents.Nat Commun. 2024 Sep 25;15(1):8193. doi: 10.1038/s41467-024-52332-3. Nat Commun. 2024. PMID: 39322632 Free PMC article.
-
Bioelectrocatalytic Cofactor Regeneration Coupled to CO2 Fixation in a Redox-Active Hydrogel for Stereoselective C-C Bond Formation.Angew Chem Int Ed Engl. 2021 Sep 13;60(38):21056-21061. doi: 10.1002/anie.202103634. Epub 2021 Jul 7. Angew Chem Int Ed Engl. 2021. PMID: 34081832 Free PMC article.
-
Connecting Biological and Synthetic Approaches for Electrocatalytic CO2 Reduction.Angew Chem Int Ed Engl. 2024 Feb 19;63(8):e202310547. doi: 10.1002/anie.202310547. Epub 2023 Dec 12. Angew Chem Int Ed Engl. 2024. PMID: 37983571 Free PMC article. Review.
-
Electrochemical Nanoreactor Provides a Comprehensive View of Isocitrate Dehydrogenase Cancer-drug Kinetics.Angew Chem Weinheim Bergstr Ger. 2023 Oct 16;135(42):e202309149. doi: 10.1002/ange.202309149. Epub 2023 Sep 12. Angew Chem Weinheim Bergstr Ger. 2023. PMID: 38529044 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

