Criteria for identifying residual tumours after neoadjuvant chemotherapy of breast cancers: a magnetic resonance imaging study
- PMID: 33436702
- PMCID: PMC7804856
- DOI: 10.1038/s41598-020-79743-8
Criteria for identifying residual tumours after neoadjuvant chemotherapy of breast cancers: a magnetic resonance imaging study
Abstract
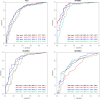
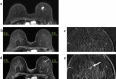
We investigated magnetic resonance imaging (MRI) criteria identifying residual tumours in patients with triple-negative and human epidermal growth factor receptor type 2-positive (HER2+) breast cancer following neoadjuvant chemotherapy. Retrospectively, 290 patients were included who had undergone neoadjuvant chemotherapy and definitive surgery. Clinicopathological features, as well as lesion size and lesion-to-background parenchymal signal enhancement ratio (SER) in early- and late-phase MRIs, were analysed. Receiver operating characteristic (ROC) analyses evaluated diagnostic performances. Maximal MRI values showing over 90% sensitivity and negative predictive value (NPV) were set as cut-off points. Identified MRI criteria were prospectively applied to 13 patients with hormone receptor-negative (HR-) tumours. The lesion size in HR-HER2-tumours had the highest area under the ROC curve value (0.92), whereas this parameter in HR + HER2 + tumours was generally low (≤ 0.75). For HR-tumours, both sensitivity and NPV exceeded the 90% threshold for early size > 0.2 cm (HR-HER2-) or > 0.1 cm (HR-HER2 +), late size > 0.4 cm, and early SER > 1.3. In the prospective pilot cohort, the criteria size and early SER did not find false negative cases, but one case was false negative with late SER. Distinguishing residual tumours based on MRI is feasible in selected triple-negative and HER2 + breast cancer patients.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Magnetic Resonance Imaging (MRI) Assessment of Residual Breast Cancer After Neoadjuvant Chemotherapy: Relevance to Tumor Subtypes and MRI Interpretation Threshold.Clin Breast Cancer. 2018 Dec;18(6):459-467.e1. doi: 10.1016/j.clbc.2018.05.009. Epub 2018 Jun 7. Clin Breast Cancer. 2018. PMID: 29954674
-
Contrast-enhanced MRI after neoadjuvant chemotherapy of breast cancer: lesion-to-background parenchymal signal enhancement ratio for discriminating pathological complete response from minimal residual tumour.Eur Radiol. 2018 Jul;28(7):2986-2995. doi: 10.1007/s00330-017-5251-8. Epub 2018 Jan 29. Eur Radiol. 2018. PMID: 29380033
-
Residual Mammographic Microcalcifications and Enhancing Lesions on MRI After Neoadjuvant Systemic Chemotherapy for Locally Advanced Breast Cancer: Correlation with Histopathologic Residual Tumor Size.Ann Surg Oncol. 2016 Apr;23(4):1135-42. doi: 10.1245/s10434-015-4993-2. Epub 2015 Dec 1. Ann Surg Oncol. 2016. PMID: 26628432
-
Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy.J Natl Cancer Inst. 2013 Mar 6;105(5):321-33. doi: 10.1093/jnci/djs528. Epub 2013 Jan 7. J Natl Cancer Inst. 2013. PMID: 23297042 Review.
-
Biomarkers of neoadjuvant/adjuvant chemotherapy for breast cancer.Chin Clin Oncol. 2020 Jun;9(3):27. doi: 10.21037/cco.2020.01.06. Epub 2020 Mar 13. Chin Clin Oncol. 2020. PMID: 32192349 Review.
Cited by
-
Efficacy Evaluation of Neoadjuvant Chemotherapy in Breast Cancer by MRI.Contrast Media Mol Imaging. 2022 Aug 4;2022:4542288. doi: 10.1155/2022/4542288. eCollection 2022. Contrast Media Mol Imaging. 2022. PMID: 36017018 Free PMC article.
-
Patient-Related Characteristics Associated with Treatment Modifications and Suboptimal Relative Dose Intensity of Neoadjuvant Chemotherapy in Patients with Breast Cancer-A Retrospective Study.Cancers (Basel). 2023 Apr 26;15(9):2483. doi: 10.3390/cancers15092483. Cancers (Basel). 2023. PMID: 37173949 Free PMC article.
-
The Role of Deep Learning in Advancing Breast Cancer Detection Using Different Imaging Modalities: A Systematic Review.Cancers (Basel). 2022 Oct 29;14(21):5334. doi: 10.3390/cancers14215334. Cancers (Basel). 2022. PMID: 36358753 Free PMC article. Review.
-
Harnessing Artificial Intelligence to Enhance Global Breast Cancer Care: A Scoping Review of Applications, Outcomes, and Challenges.Cancers (Basel). 2025 Jan 9;17(2):197. doi: 10.3390/cancers17020197. Cancers (Basel). 2025. PMID: 39857979 Free PMC article. Review.
-
Assessment and Prediction of Response to Neoadjuvant Chemotherapy in Breast Cancer: A Comparison of Imaging Modalities and Future Perspectives.Cancers (Basel). 2021 Jul 14;13(14):3521. doi: 10.3390/cancers13143521. Cancers (Basel). 2021. PMID: 34298733 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

