Double transition metal MXene (TixTa4-xC3) 2D materials as anodes for Li-ion batteries
- PMID: 33436822
- PMCID: PMC7804453
- DOI: 10.1038/s41598-020-79991-8
Double transition metal MXene (TixTa4-xC3) 2D materials as anodes for Li-ion batteries
Erratum in
-
Author Correction: Double transition metal MXene (TixTa4-xC3) 2D materials as anodes for Li-ion batteries.Sci Rep. 2021 Jun 14;11(1):12862. doi: 10.1038/s41598-021-92167-2. Sci Rep. 2021. PMID: 34127767 Free PMC article. No abstract available.
Abstract
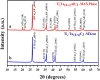
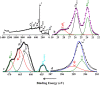
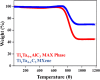
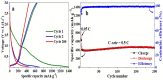
A bi-metallic titanium-tantalum carbide MXene, TixTa(4-x)C3 is successfully prepared via etching of Al atoms from parent TixTa(4-x)AlC3 MAX phase for the first time. X-ray diffractometer and Raman spectroscopic analysis proved the crystalline phase evolution from the MAX phase to the lamellar MXene arrangements. Also, the X-ray photoelectron spectroscopy (XPS) study confirmed that the synthesized MXene is free from Al after hydro fluoric acid (HF) etching process as well as partial oxidation of Ti and Ta. Moreover, the FE-SEM and TEM characterizations demonstrate the exfoliation process tailored by the TixTa(4-x)C3 MXene after the Al atoms from its corresponding MAX TixTa(4-x)AlC3 phase, promoting its structural delamination with an expanded interlayer d-spacing, which can allow an effective reversible Li-ion storage. The lamellar TixTa(4-x)C3 MXene demonstrated a reversible specific discharge capacity of 459 mAhg-1 at an applied C-rate of 0.5 °C with a capacity retention of 97% over 200 cycles. An excellent electrochemical redox performance is attributed to the formation of a stable, promising bi-metallic MXene material, which stores Li-ions on the surface of its layers. Furthermore, the TixTa(4-x)C3 MXene anode demonstrate a high rate capability as a result of its good electron and Li-ion transport, suggesting that it is a promising candidate as Li-ion anode material.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Ultra-Efficient Synthesis of Nb4 C3 Tx MXene via H2 O-Assisted Supercritical Etching for Li-Ion Battery.Small Methods. 2024 Mar;8(3):e2300836. doi: 10.1002/smtd.202300836. Epub 2023 Nov 5. Small Methods. 2024. PMID: 37926701
-
Flexible Mn3O4/MXene Films with 2D-2D Architectures as Stable and Ultrafast Anodes for Li-Ion Batteries.ACS Appl Mater Interfaces. 2022 Oct 19;14(41):46502-46512. doi: 10.1021/acsami.2c11577. Epub 2022 Oct 4. ACS Appl Mater Interfaces. 2022. PMID: 36194645
-
Construction of two-dimensional bimetal (Fe-Ti) oxide/carbon/MXene architecture from titanium carbide MXene for ultrahigh-rate lithium-ion storage.J Colloid Interface Sci. 2021 Apr 15;588:147-156. doi: 10.1016/j.jcis.2020.12.071. Epub 2020 Dec 31. J Colloid Interface Sci. 2021. PMID: 33388580
-
Nitrogen and sulfur co-doped vanadium carbide MXene for highly reversible lithium-ion storage.J Colloid Interface Sci. 2021 Apr;587:489-498. doi: 10.1016/j.jcis.2020.12.044. Epub 2020 Dec 17. J Colloid Interface Sci. 2021. PMID: 33387843
-
Construction of 2D sandwich-like Na2V6O16·3H2O@MXene heterostructure for advanced aqueous zinc ion batteries.J Colloid Interface Sci. 2024 Feb;655:226-233. doi: 10.1016/j.jcis.2023.11.020. Epub 2023 Nov 4. J Colloid Interface Sci. 2024. PMID: 37944370
Cited by
-
Atomic Scale Design of MXenes and Their Parent Materials─From Theoretical and Experimental Perspectives.Chem Rev. 2023 Dec 13;123(23):13291-13322. doi: 10.1021/acs.chemrev.3c00241. Epub 2023 Nov 17. Chem Rev. 2023. PMID: 37976459 Free PMC article. Review.
-
Development of Fluorine-Free Tantalum Carbide MXene Hybrid Structure as a Biocompatible Material for Supercapacitor Electrodes.Adv Funct Mater. 2021 Jul 23;31(30):2100015. doi: 10.1002/adfm.202100015. Epub 2021 May 24. Adv Funct Mater. 2021. PMID: 35264918 Free PMC article.
-
Beyond graphene: exploring the potential of MXene anodes for enhanced lithium-sulfur battery performance.RSC Adv. 2024 Jun 21;14(28):20032-20047. doi: 10.1039/d4ra02704c. eCollection 2024 Jun 18. RSC Adv. 2024. PMID: 38911835 Free PMC article. Review.
-
Recent Advances in Non-Ti MXenes: Synthesis, Properties, and Novel Applications.Adv Sci (Weinh). 2024 Sep;11(36):e2303998. doi: 10.1002/advs.202303998. Epub 2024 Jun 18. Adv Sci (Weinh). 2024. PMID: 38894594 Free PMC article. Review.
-
MXene Contact Engineering for Printed Electronics.Adv Sci (Weinh). 2023 Jul;10(19):e2207174. doi: 10.1002/advs.202207174. Epub 2023 Apr 25. Adv Sci (Weinh). 2023. PMID: 37096843 Free PMC article. Review.
References
-
- Deng Y, Wan L, Xie Y, Qin X, Chen G. Recent advances in Mn-based oxides as anode materials for lithium ion batteries. RSC Adv. 2014;4:23914–23935.
-
- Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004;104:4303–4417. - PubMed
-
- Ozawa K. Lithium-ion rechargeable batteries with LiCoO2 and carbon electrodes: the LiCoO2/C system. Solid State Ionics. 1994;69:212–221.
-
- Kato, H., Yamamoto, Y., Nagamine, M. & Nishi, Y. Lithium ion rechargeable batteries. In Proc. WESCON ’93, Vol. 51, 210–214 (IEEE, 1993).
-
- Brar VW, Koltonow AR, Huang J. New discoveries and opportunities from two-dimensional materials. ACS Photonics. 2017;4:407–411.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

