Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells
- PMID: 33436830
- PMCID: PMC7804192
- DOI: 10.1038/s41598-020-80302-4
Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells
Abstract
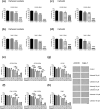
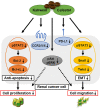
Despite improvements in systemic therapy options for renal cancer, it remains one of the most drug-resistant malignancies. Interestingly, reports have shown that kahweol and cafestol, natural diterpenes extracted from coffee beans, exhibit anti-cancer activity. However, the multiple potential pharmacological actions of both have yet to be fully understood. This study therefore investigated the effects of kahweol acetate and cafestol on human renal cancer ACHN and Caki-1 cells. Accordingly, the combination of kahweol acetate and cafestol administration synergistically inhibited cell proliferation and migration by inducing apoptosis and inhibiting epithelial-mesenchymal transition. Mechanistic dissection revealed that kahweol acetate and cafestol inhibited Akt and ERK phosphorylation. Moreover, kahweol acetate and cafestol downregulated the expression of not only C-C chemokine receptors 2, 5, and 6 but also programmed death-ligand 1, indicating their effects on the tumor microenvironment. Thus, kahweol acetate and cafestol may be novel therapeutic candidates for renal cancer considering that they exert multiple pharmacological effects.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Recent Updates on the Functional Impact of Kahweol and Cafestol on Cancer.Molecules. 2022 Oct 28;27(21):7332. doi: 10.3390/molecules27217332. Molecules. 2022. PMID: 36364160 Free PMC article. Review.
-
Coffee diterpenes kahweol acetate and cafestol synergistically inhibit the proliferation and migration of prostate cancer cells.Prostate. 2019 Apr;79(5):468-479. doi: 10.1002/pros.23753. Epub 2018 Dec 19. Prostate. 2019. PMID: 30569541
-
Cafestol overcomes ABT-737 resistance in Mcl-1-overexpressed renal carcinoma Caki cells through downregulation of Mcl-1 expression and upregulation of Bim expression.Cell Death Dis. 2014 Nov 6;5(11):e1514. doi: 10.1038/cddis.2014.472. Cell Death Dis. 2014. PMID: 25375379 Free PMC article.
-
Natural diterpenes from coffee, cafestol and kahweol induce apoptosis through regulation of specificity protein 1 expression in human malignant pleural mesothelioma.J Biomed Sci. 2012 Jun 26;19(1):60. doi: 10.1186/1423-0127-19-60. J Biomed Sci. 2012. PMID: 22734486 Free PMC article.
-
Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties.Int J Mol Sci. 2019 Aug 30;20(17):4238. doi: 10.3390/ijms20174238. Int J Mol Sci. 2019. PMID: 31480213 Free PMC article. Review.
Cited by
-
Recent Updates on the Functional Impact of Kahweol and Cafestol on Cancer.Molecules. 2022 Oct 28;27(21):7332. doi: 10.3390/molecules27217332. Molecules. 2022. PMID: 36364160 Free PMC article. Review.
-
Nanoemulsions of Phoenix dactylifera L. (Decaffeinated) and Coffea arabica L. Extracts as a Novel Approach for the Treatment of Carbon Tetrachloride-Mediated Liver Fibrosis.Antioxidants (Basel). 2024 Mar 16;13(3):355. doi: 10.3390/antiox13030355. Antioxidants (Basel). 2024. PMID: 38539888 Free PMC article.
-
A Decade of Research on Coffee as an Anticarcinogenic Beverage.Oxid Med Cell Longev. 2021 Sep 15;2021:4420479. doi: 10.1155/2021/4420479. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34567408 Free PMC article. Review.
-
Toxicological Risk Assessment of Coffee Oil (Coffee Seed Oil and Spent Coffee Grounds Oil) as a Novel Food with Focus on Cafestol.Molecules. 2025 Jul 12;30(14):2951. doi: 10.3390/molecules30142951. Molecules. 2025. PMID: 40733217 Free PMC article. Review.
-
Unraveling Connective Tissue Growth Factor as a Therapeutic Target and Assessing Kahweol as a Potential Drug Candidate in Triple-Negative Breast Cancer Treatment.Int J Mol Sci. 2023 Nov 14;24(22):16307. doi: 10.3390/ijms242216307. Int J Mol Sci. 2023. PMID: 38003505 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

