Chronic stress and corticosterone exacerbate alcohol-induced tissue injury in the gut-liver-brain axis
- PMID: 33436875
- PMCID: PMC7804442
- DOI: 10.1038/s41598-020-80637-y
Chronic stress and corticosterone exacerbate alcohol-induced tissue injury in the gut-liver-brain axis
Abstract
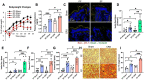
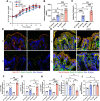
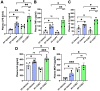
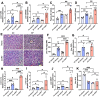
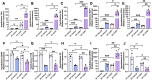
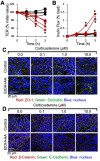
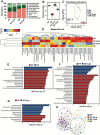
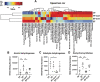
Alcohol use disorders are associated with altered stress responses, but the impact of stress or stress hormones on alcohol-associated tissue injury remain unknown. We evaluated the effects of chronic restraint stress on alcohol-induced gut barrier dysfunction and liver damage in mice. To determine whether corticosterone is the stress hormone associated with the stress-induced effects, we evaluated the effect of chronic corticosterone treatment on alcoholic tissue injury at the Gut-Liver-Brain (GLB) axis. Chronic restraint stress synergized alcohol-induced epithelial tight junction disruption and mucosal barrier dysfunction in the mouse intestine. These effects of stress on the gut were reproduced by corticosterone treatment. Corticosterone synergized alcohol-induced expression of inflammatory cytokines and chemokines in the colonic mucosa, and it potentiated the alcohol-induced endotoxemia and systemic inflammation. Corticosterone also potentiated alcohol-induced liver damage and neuroinflammation. Metagenomic analyses of 16S RNA from fecal samples indicated that corticosterone modulates alcohol-induced changes in the diversity and abundance of gut microbiota. In Caco-2 cell monolayers, corticosterone dose-dependently potentiated ethanol and acetaldehyde-induced tight junction disruption and barrier dysfunction. These data indicate that chronic stress and corticosterone exacerbate alcohol-induced mucosal barrier dysfunction, endotoxemia, and systemic alcohol responses. Corticosterone-mediated promotion of alcohol-induced intestinal epithelial barrier dysfunction and modulation of gut microbiota may play a crucial role in the mechanism of stress-induced promotion of alcohol-associated tissue injury at the GLB axis.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Central role of intestinal epithelial glucocorticoid receptor in alcohol- and corticosterone-induced gut permeability and systemic response.FASEB J. 2022 Jan;36(1):e22061. doi: 10.1096/fj.202101424R. FASEB J. 2022. PMID: 34861075 Free PMC article.
-
TRPV6 deficiency attenuates stress and corticosterone-mediated exacerbation of alcohol-induced gut barrier dysfunction and systemic inflammation.Front Immunol. 2023 Jan 31;14:1093584. doi: 10.3389/fimmu.2023.1093584. eCollection 2023. Front Immunol. 2023. PMID: 36817471 Free PMC article.
-
EGF receptor plays a role in the mechanism of glutamine-mediated prevention of alcohol-induced gut barrier dysfunction and liver injury.J Nutr Biochem. 2019 Feb;64:128-143. doi: 10.1016/j.jnutbio.2018.10.016. Epub 2018 Nov 6. J Nutr Biochem. 2019. PMID: 30502657 Free PMC article.
-
Alcoholic liver injury: influence of gender and hormones.World J Gastroenterol. 2010 Mar 21;16(11):1377-84. doi: 10.3748/wjg.v16.i11.1377. World J Gastroenterol. 2010. PMID: 20238405 Free PMC article. Review.
-
Chronic stress-induced gut dysfunction exacerbates Parkinson's disease phenotype and pathology in a rotenone-induced mouse model of Parkinson's disease.Neurobiol Dis. 2020 Feb;135:104352. doi: 10.1016/j.nbd.2018.12.012. Epub 2018 Dec 21. Neurobiol Dis. 2020. PMID: 30579705 Review.
Cited by
-
Gut-liver axis: Recent concepts in pathophysiology in alcohol-associated liver disease.Hepatology. 2024 Dec 1;80(6):1342-1371. doi: 10.1097/HEP.0000000000000924. Epub 2024 May 1. Hepatology. 2024. PMID: 38691396 Review.
-
Examination of Physiological and Morphological Differences between Farm-Bred and Wild Black-Spotted Pond Frogs (Pelophylax nigromaculatus).Life (Basel). 2021 Oct 15;11(10):1089. doi: 10.3390/life11101089. Life (Basel). 2021. PMID: 34685460 Free PMC article.
-
Paneth cell dysfunction in radiation injury and radio-mitigation by human α-defensin 5.Front Immunol. 2023 Aug 10;14:1174140. doi: 10.3389/fimmu.2023.1174140. eCollection 2023. Front Immunol. 2023. PMID: 37638013 Free PMC article.
-
Acetobacter pasteurianus BP2201 alleviates alcohol-induced hepatic and neuro-toxicity and modulate gut microbiota in mice.Microb Biotechnol. 2023 Sep;16(9):1834-1857. doi: 10.1111/1751-7915.14303. Epub 2023 Jun 24. Microb Biotechnol. 2023. PMID: 37354051 Free PMC article.
-
Rhizophora mucronata Lam. (Mangrove) Bark Extract Reduces Ethanol-Induced Liver Cell Death and Oxidative Stress in Swiss Albino Mice: In Vivo and In Silico Studies.Metabolites. 2022 Oct 25;12(11):1021. doi: 10.3390/metabo12111021. Metabolites. 2022. PMID: 36355104 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

