Antimicrobial activity of IDD-B40 against drug-resistant Mycobacterium tuberculosis
- PMID: 33436895
- PMCID: PMC7804135
- DOI: 10.1038/s41598-020-80227-y
Antimicrobial activity of IDD-B40 against drug-resistant Mycobacterium tuberculosis
Abstract
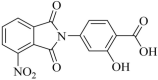
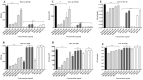
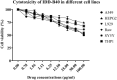
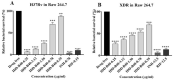
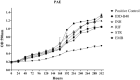
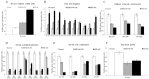
The emergence of multi-drug resistant (MDR) and extensively drug-resistant (XDR) Mycobacterium tuberculosis creates the urgency for new anti-tuberculosis drugs to improve the efficiency of current tuberculosis treatment. In the search for a new potential tuberculosis drug, we synthesized an isoindole based chemical library and screened a potential candidate with significant anti-tuberculosis activity. The compound named 2-hydroxy-4-(4-nitro-1,3-dioxoisoindolin-2-yl) benzoic acid (IDD-B40) showed strong activity against all the tested drug-susceptible and drug-resistant strains of M. tuberculosis, with the 50% minimum inhibitory concentrations (MIC50) of 0.39 μg/ml both in culture broth and inside Raw 264.7 cells. Also, IDD-B40, in combination with rifampicin, exhibited a direct synergistic effect against both XDR and H37Rv M. tuberculosis. Besides, IDD-B40 showed a better post-antibiotic effect (PAE) than did some first-line drugs and showed no significant cytotoxicity to any cell line tested, with a selectivity index of ≥ 128. Although IDD-B40 showed a result similar to isoniazid in the preliminary mycolic acid inhibition assay, it did not exhibit any effect against other mycolic acid-producing nontuberculous mycobacterial strains (NTM), and different non-mycobacterial pathogenic strains, so further studies are required to confirm the mode of action of IDD-B40. Considering its results against M. tuberculosis, IDD-B40 is a potential anti-tuberculosis drug candidate. However, further studies are required to evaluate its potential in vivo effect and therapeutic potential.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO . Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

