Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration
- PMID: 33436904
- PMCID: PMC7804460
- DOI: 10.1038/s41598-020-80608-3
Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration
Abstract
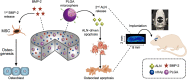
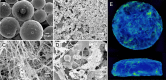
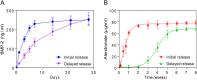
The clinical use of bioactive molecules in bone regeneration has been known to have side effects, which result from uncontrolled and supraphysiological doses. In this study, we demonstrated the synergistic effect of two bioactive molecules, bone morphogenic protein-2 (BMP-2) and alendronate (ALN), by releasing them in a sequential manner. Collagen-hydroxyapatite composite scaffolds functionalized using BMP-2 are loaded with biodegradable microspheres where ALN is encapsulated. The results indicate an initial release of BMP-2 for a few days, followed by the sequential release of ALN after two weeks. The composite scaffolds significantly increase osteogenic activity owing to the synergistic effect of BMP-2 and ALN. Enhanced bone regeneration was identified at eight weeks post-implantation in the rat 8-mm critical-sized defect. Our findings suggest that the sequential delivery of BMP-2 and ALN from the scaffolds results in a synergistic effect on bone regeneration, which is unprecedented. Therefore, such a system exhibits potential for the application of cell-free tissue engineering.
Conflict of interest statement
The authors declare no competing interests.
Figures


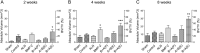
Similar articles
-
Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration.Biomaterials. 2016 Nov;106:205-16. doi: 10.1016/j.biomaterials.2016.08.023. Epub 2016 Aug 17. Biomaterials. 2016. PMID: 27566869
-
The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model.Int J Mol Sci. 2015 Nov 6;16(11):26738-53. doi: 10.3390/ijms161125982. Int J Mol Sci. 2015. PMID: 26561810 Free PMC article.
-
Evaluation of BMP-2 and VEGF loaded 3D printed hydroxyapatite composite scaffolds with enhanced osteogenic capacity in vitro and in vivo.Mater Sci Eng C Mater Biol Appl. 2020 Jul;112:110893. doi: 10.1016/j.msec.2020.110893. Epub 2020 Mar 21. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32409051
-
Novel Strategies for Spatiotemporal and Controlled BMP-2 Delivery in Bone Tissue Engineering.Cell Transplant. 2024 Jan-Dec;33:9636897241276733. doi: 10.1177/09636897241276733. Cell Transplant. 2024. PMID: 39305020 Free PMC article. Review.
-
Collagen-based biocomposites inspired by bone hierarchical structures for advanced bone regeneration: ongoing research and perspectives.Biomater Sci. 2022 Jan 18;10(2):318-353. doi: 10.1039/d1bm01294k. Biomater Sci. 2022. PMID: 34783809 Review.
Cited by
-
Effect of recombinant human bone morphogenetic protein-2 and osteoprotegerin-Fc in MC3T3-E1 cells.J Rheum Dis. 2024 Apr 1;31(2):79-85. doi: 10.4078/jrd.2023.0043. Epub 2024 Feb 1. J Rheum Dis. 2024. PMID: 38559798 Free PMC article.
-
Natural bone-mimicking nanopore-incorporated hydroxyapatite scaffolds for enhanced bone tissue regeneration.Biomater Res. 2022 Feb 25;26(1):7. doi: 10.1186/s40824-022-00253-x. Biomater Res. 2022. PMID: 35216625 Free PMC article.
-
Optimizing Delivery of Therapeutic Growth Factors for Bone and Cartilage Regeneration.Gels. 2023 May 3;9(5):377. doi: 10.3390/gels9050377. Gels. 2023. PMID: 37232969 Free PMC article. Review.
-
Nanoindentation for Monitoring the Time-Variant Mechanical Strength of Drug-Loaded Collagen Hydrogel Regulated by Hydroxyapatite Nanoparticles.ACS Omega. 2021 Mar 23;6(13):9269-9278. doi: 10.1021/acsomega.1c00824. eCollection 2021 Apr 6. ACS Omega. 2021. PMID: 33842796 Free PMC article.
-
How Is Bone Regeneration Influenced by Polymer Membranes? Insight into the Histological and Radiological Point of View in the Literature.Membranes (Basel). 2024 Sep 11;14(9):193. doi: 10.3390/membranes14090193. Membranes (Basel). 2024. PMID: 39330534 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

