Evaluation for pharmacokinetic exposure of cytotoxic anticancer drugs in elderly patients receiving (R-)CHOP therapy
- PMID: 33436910
- PMCID: PMC7803984
- DOI: 10.1038/s41598-020-80706-2
Evaluation for pharmacokinetic exposure of cytotoxic anticancer drugs in elderly patients receiving (R-)CHOP therapy
Abstract
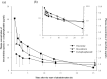
(R-)miniCHOP therapy, which delivers approximately half-doses of the (R-)CHOP regimen, has shown efficacy and safety in patients who are more than 80 years old. This study aimed to compare the area under the plasma concentration-time curves (AUCs) of vincristine (VCR), doxorubicin (DXR), and cyclophosphamide (CPA) between (R-)CHOP and (R-)miniCHOP regimens. The AUCs were compared between patients aged 65-79 years receiving (R-)CHOP therapy and those aged 80 years and older receiving (R-)miniCHOP therapy. Age was not an independent variable for predicting the dose-adjusted AUCs (AUC/Ds) of cytotoxic anticancer drugs. The median AUCs of DXR and CPA were significantly smaller in the (R-)miniCHOP group than in the (R-)CHOP group (168.7 vs. 257.9 ng h/mL, P = 0.003, and 219.9 vs. 301.7 µg h/mL, P = 0.020, respectively). The median AUCs of VCR showed the same trend but the difference was not significant (24.83 vs. 34.85 ng h/mL, P = 0.135). It is possible that the AUCs of VCR, DXR, and CPA in patients aged 80 years and older receiving (R-)miniCHOP therapy may be lower than those in patients 65-79 years old receiving (R-)CHOP therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Cyclophosphamide dose adjustment based on body weight and albuminemia in elderly patients treated with R-mini-CHOP.Cancer Chemother Pharmacol. 2019 Apr;83(4):775-785. doi: 10.1007/s00280-019-03775-9. Epub 2019 Jan 28. Cancer Chemother Pharmacol. 2019. PMID: 30689002
-
Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial.Lancet Oncol. 2011 May;12(5):460-8. doi: 10.1016/S1470-2045(11)70069-9. Epub 2011 Apr 7. Lancet Oncol. 2011. PMID: 21482186 Clinical Trial.
-
Influence of dose reduction of vincristine in R-CHOP on outcomes of diffuse large B cell lymphoma.Ann Hematol. 2016 Jan;95(1):41-47. doi: 10.1007/s00277-015-2514-9. Epub 2015 Oct 5. Ann Hematol. 2016. PMID: 26435364
-
Frontline treatment of diffuse large B-cell lymphoma in elderly: a systematic review of clinical trials in post-rituximab era.Leuk Lymphoma. 2018 Dec;59(12):2847-2861. doi: 10.1080/10428194.2018.1443332. Epub 2018 Apr 4. Leuk Lymphoma. 2018. PMID: 29616868
-
Evolution of R-CHOP therapy for older patients with diffuse large B-cell lymphoma.Expert Rev Anticancer Ther. 2008 Oct;8(10):1651-8. doi: 10.1586/14737140.8.10.1651. Expert Rev Anticancer Ther. 2008. PMID: 18925856 Review.
Cited by
-
Influence of CYP3A5 and ABCB1 polymorphisms on the pharmacokinetics of vincristine in adult patients receiving CHOP therapy.Cancer Chemother Pharmacol. 2023 Nov;92(5):391-398. doi: 10.1007/s00280-023-04580-1. Epub 2023 Aug 23. Cancer Chemother Pharmacol. 2023. PMID: 37610625
-
Screening for Prognostic microRNAs Associated with Treatment Failure in Diffuse Large B Cell Lymphoma.Cancers (Basel). 2022 Feb 20;14(4):1065. doi: 10.3390/cancers14041065. Cancers (Basel). 2022. PMID: 35205813 Free PMC article.
-
A cell culture system to model pharmacokinetics using adjustable-volume perfused mixing chambers.Toxicol In Vitro. 2023 Sep;91:105623. doi: 10.1016/j.tiv.2023.105623. Epub 2023 May 24. Toxicol In Vitro. 2023. PMID: 37236431 Free PMC article.
References
-
- Pfreundschuh M, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–391. doi: 10.1016/S1470-2045(06)70664-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

