Probiotic lactobacilli as a promising strategy to ameliorate disorders associated with intestinal inflammation induced by a non-steroidal anti-inflammatory drug
- PMID: 33436961
- PMCID: PMC7803994
- DOI: 10.1038/s41598-020-80482-z
Probiotic lactobacilli as a promising strategy to ameliorate disorders associated with intestinal inflammation induced by a non-steroidal anti-inflammatory drug
Abstract
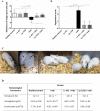
Damage to the small intestine caused by non-steroidal anti-inflammatory drugs (NSAIDs) occurs more frequently than in the upper gastrointestinal tract, is more difficult to diagnose and no effective treatments exist. Hence, we investigated whether probiotics can control the onset of this severe condition in a murine model of intestinal inflammation induced by the NSAID, indomethacin. Probiotic supplementation to mice reduce the body weight loss, anemia, shortening of the small intestine, cell infiltration into the intestinal tissue and the loss of Paneth and Goblet cells associated with intestinal inflammation. Furthermore, a high antimicrobial activity in the intestinal fluids of mice fed with probiotics compared to animals on a conventional diet was elicited against several pathogens. Interestingly, probiotics dampened the oxidative stress and several local and systemic markers of an inflammatory process, as well as increased the secretion of IL-10 by regulatory T cells. Even more importantly, probiotics induced important changes in the large intestine microbiota characterized by an increase in anaerobes and lactobacilli, and a significant decrease in total enterobacteria. We conclude that oral probiotic supplementation in NSAID-induced inflammation increases intestinal antimicrobial activity and reinforces the intestinal epithelial barrier in order to avoid pathogens and commensal invasion and maintain intestinal homeostasis.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

