Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2
- PMID: 33436985
- PMCID: PMC7804421
- DOI: 10.1038/s41598-020-80896-9
Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2
Abstract
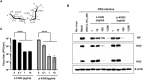
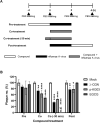
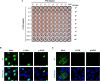
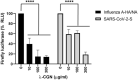
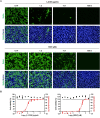
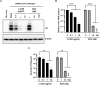
Influenza virus and coronavirus, belonging to enveloped RNA viruses, are major causes of human respiratory diseases. The aim of this study was to investigate the broad spectrum antiviral activity of a naturally existing sulfated polysaccharide, lambda-carrageenan (λ-CGN), purified from marine red algae. Cell culture-based assays revealed that the macromolecule efficiently inhibited both influenza A and B viruses with EC50 values ranging from 0.3 to 1.4 μg/ml, as well as currently circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with an EC50 value of 0.9 ± 1.1 μg/ml. No toxicity to the host cells was observed at concentrations up to 300 μg/ml. Plaque titration and western blot analysis verified that λ-CGN reduced expression of viral proteins in cell lysates and suppressed progeny virus production in culture supernatants in a dose-dependent manner. This polyanionic compound exerts antiviral activity by targeting viral attachment to cell surface receptors and preventing virus entry. Moreover, its intranasal administration to mice during influenza A viral challenge not only alleviated infection-mediated reductions in body weight but also protected 60% of mice from virus-induced mortality. Thus, λ-CGN could be a promising antiviral agent for preventing infection with several respiratory viruses.
Conflict of interest statement
Y.J., H.S., M.K.L, O.S.K., J.S.S., C.W.K., H.-R.L., and M.K. declare no conflict of interest. Y.K. is trying to commercialize λ-CGN used in this study through the company Hanmi Pharmaceutical Co.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

